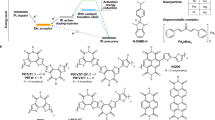
Abstract
The chemical doping of molecular semiconductors is based on electron-transfer reactions between the semiconductor and dopant molecules; here, the redox potential of the dopant is key to control the Fermi level of the semiconductor1,2. The tunability and reproducibility of chemical doping are limited by the availability of dopant materials and the effects of impurities such as water. Here we focused on proton-coupled electron-transfer (PCET) reactions, which are widely used in biochemical processes3,4; their redox potentials depend on an easily handled parameter, that is, proton activity. We immersed p-type organic semiconductor thin films in aqueous solutions with PCET-based redox pairs and hydrophobic molecular ions. Synergistic reactions of PCET and ion intercalation resulted in efficient chemical doping of crystalline organic semiconductor thin films under ambient conditions. In accordance with the Nernst equation, the Fermi levels of the semiconductors were controlled reproducibly with a high degree of precision—a thermal energy of about 25 millielectronvolts at room temperature and over a few hundred millielectronvolts around the band edge. A reference-electrode-free, resistive pH sensor based on this method is also proposed. A connection between semiconductor doping and proton activity, a widely used parameter in chemical and biochemical processes, may help create a platform for ambient semiconductor processes and biomolecular electronics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the plots within this study are available from Zenodo at https://doi.org/10.5281/zenodo.8166159.
References
Jacobs, I. E. & Moulé, A. J. Controlling molecular doping in organic semiconductors. Adv. Mater. 29, 1703063 (2017).
Scaccabarozzi, A. D. et al. Doping approaches for organic semiconductors. Chem. Rev. 122, 4420–4492 (2021).
Efremov, R. G., Baradaran, R. & Sazanov, L. A. The architecture of respiratory complex I. Nature 465, 441–445 (2010).
Reece, S. Y. & Nocera, D. G. Proton-coupled electron transfer in biology: results from synergistic studies in natural and model systems. Annu. Rev. Biochem. 78, 673–699 (2009).
Sze, S. M. Semiconductor Devices: Physics and Technology (John Wiley & Sons, 2008).
Wang, T. et al. Transporting holes stably under iodide invasion in efficient perovskite solar cells. Science 377, 1227–1232 (2022).
Karki, A. et al. Doped semiconducting polymer nanoantennas for tunable organic plasmonics. Commun. Mater. 3, 48 (2022).
Zhao, W., Ding, J., Zou, Y., Di, C.-A. & Zhu, D. Chemical doping of organic semiconductors for thermoelectric applications. Chem. Soc. Rev. 49, 7210–7228 (2020).
Lüssem, B., Riede, M. & Leo, K. Doping of organic semiconductors. Phys. Status Solidi A 210, 9–43 (2013).
Cochran, J. E. et al. Molecular interactions and ordering in electrically doped polymers: blends of PBTTT and F4TCNQ. Macromolecules 47, 6836–6846 (2014).
Kang, K. et al. 2D coherent charge transport in highly ordered conducting polymers doped by solid state diffusion. Nat. Mater. 15, 896–902 (2016).
Untilova, V., Biskup, T., Biniek, L., Vijayakumar, V. & Brinkmann, M. Control of chain alignment and crystallization helps enhance charge conductivities and thermoelectric power factors in sequentially doped P3HT:F4TCNQ films. Macromolecules 53, 2441–2453 (2020).
Salzmann, I., Heimel, G., Oehzelt, M., Winkler, S. & Koch, N. Molecular electrical doping of organic semiconductors: fundamental mechanisms and emerging dopant design rules. Acc. Chem. Res. 49, 370–378 (2016).
De Leeuw, D., Simenon, M., Brown, A. & Einerhand, R. Stability of n-type doped conducting polymers and consequences for polymeric microelectronic devices. Synth. Met. 87, 53–59 (1997).
Guo, S. et al. n-doping of organic electronic materials using air-stable organometallics. Adv. Mater. 24, 699–703 (2012).
Zhang, Y. et al. Electron transport and nanomorphology in solution-processed polymeric semiconductor n-doped with an air-stable organometallic dimer. Adv. Electron. Mater. 3, 1600546 (2017).
Yamashita, Y. et al. Highly air-stable, n-doped conjugated polymers achieved by dimeric organometallic dopants. J. Mater. Chem. C 9, 4105–4111 (2021).
Yamashita, Y. et al. Efficient molecular doping of polymeric semiconductors driven by anion exchange. Nature 572, 634–638 (2019).
Thomas, E. M. et al. Effects of counter-ion size on delocalization of carriers and stability of doped semiconducting polymers. Adv. Electron. Mater. 6, 2000595 (2020).
Jacobs, I. E. et al. High-efficiency ion-exchange doping of conducting polymers. Adv. Mater. 34, 2102988 (2022).
Tang, C. G. et al. Doped polymer semiconductors with ultrahigh and ultralow work functions for ohmic contacts. Nature 539, 536–540 (2016).
Yurash, B. et al. Towards understanding the doping mechanism of organic semiconductors by Lewis acids. Nat. Mater. 18, 1327–1334 (2019).
Dixon, A. L., Vezin, H., Nguyen, T.-Q. & Reddy, G. M. Structural insights into Lewis acid- and F4TCNQ-doped conjugated polymers by solid-state magnetic resonance spectroscopy. Mater. Horiz. 9, 981–990 (2022).
Lin, X. et al. Beating the thermodynamic limit with photo-activation of n-doping in organic semiconductors. Nat. Mater. 16, 1209–1215 (2017).
Yang, C.-Y. et al. A thermally activated and highly miscible dopant for n-type organic thermoelectrics. Nat. Commun. 11, 3292 (2020).
Guo, H. et al. Transition metal-catalysed molecular n-doping of organic semiconductors. Nature 599, 67–73 (2021).
Huynh, M. T., Anson, C. W., Cavell, A. C., Stahl, S. S. & Hammes-Schiffer, S. Quinone 1e− and 2e−/2H+ reduction potentials: identification and analysis of deviations from systematic scaling relationships. J. Am. Chem. Soc. 138, 15903–15910 (2016).
Ding, Y., Li, Y. & Yu, G. Exploring bio-inspired quinone-based organic redox flow batteries: a combined experimental and computational study. Chem 1, 790–801 (2016).
Bratsch, S. G. Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data 18, 1–21 (1989).
Trasatti, S. The absolute electrode potential: an explanatory note (recommendations 1986). Pure Appl. Chem. 58, 955–966 (1986).
Hayes, J. C. & Lietzke, M. The standard electrode potential of the quinhydrone electrode from 25 to 55°. J. Phys. Chem. 64, 374–376 (1960).
McCulloch, I. et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 5, 328–333 (2006).
Yamashita, Y. et al. Supramolecular cocrystals built through redox-triggered ion intercalation in π-conjugated polymers. Commun. Mater. 2, 45 (2021).
Vijayakumar, V. et al. Effect of alkyl side chain length on doping kinetics, thermopower, and charge transport properties in highly oriented F4TCNQ-doped PBTTT films. ACS Appl. Mater. Interfaces 11, 4942–4953 (2019).
Bischak, C. G., Flagg, L. Q. & Ginger, D. S. Ion exchange gels allow organic electrochemical transistor operation with hydrophobic polymers in aqueous solution. Adv. Mater. 32, 2002610 (2020).
Koh, Q.-M. et al. Overcoming the water oxidative limit for ultra-high-workfunction hole-doped polymers. Nat. Commun. 12, 3345 (2021).
Hofmann, A. I., Kroon, R., Yu, L. & Müller, C. Highly stable doping of a polar polythiophene through co-processing with sulfonic acids and bistriflimide. J. Mater. Chem. C 6, 6905–6910 (2018).
Cendra, C. et al. Role of the anion on the transport and structure of organic mixed conductors. Adv. Funct. Mater. 29, 1807034 (2019).
Flagg, L. Q., Giridharagopal, R., Guo, J. & Ginger, D. S. Anion-dependent doping and charge transport in organic electrochemical transistors. Chem. Mater. 30, 5380–5389 (2018).
Cho, E. et al. Three-dimensional packing structure and electronic properties of biaxially oriented poly(2,5-bis(3-alkylthiophene-2-yl)thieno[3,2-b]thiophene) films. J. Am. Chem. Soc. 134, 6177–6190 (2012).
Guardado, J. O. & Salleo, A. Structural effects of gating poly(3-hexylthiophene) through an ionic liquid. Adv. Funct. Mater. 27, 1701791 (2017).
Reyes Cruz, E. A. et al. Molecular-modified photocathodes for applications in artificial photosynthesis and solar-to-fuel technologies. Chem. Rev. 122, 16051–16109 (2022).
Boettcher, S. W. et al. Potentially confusing: potentials in electrochemistry. ACS Energy Lett. 6, 261–266 (2020).
Acknowledgements
M.I. was supported by JST, the establishment of university fellowships towards the creation of science technology innovation (grant number JPMJFS2144). This work was supported in part by JSPS KAKENHI grants (numbers JP20K15358, JP20H00392, JP22H02160 and JP22H04959). This work was supported in part by JST, CREST grant number JPMJCR21O3, Japan.
Author information
Authors and Affiliations
Contributions
M.I. and Y.Y. conceived, designed and performed the experiments, and analysed the data. M.I. and Y.Y. wrote the paper. S.W., K.A. and J.T. supervised the work. All authors discussed the results and reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Xavier Crispin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Effects of dissolved oxygen on the p-type doping of PBTTT thin films.
a, Conductivities of PBTTT thin films immersed in aqueous solutions with LiTFSI at pH 1-4. Solution pH was adjusted by H2SO4 and KOH. The error bars indicate uncertainties in the conductivity mainly stemming from uncertainties in the thickness of the PBTTT thin films and represent 1 s.d. among four individual samples. The markers indicate average values. b, UV-vis-NIR spectra of PBTTT thin films immersed in aqueous solutions with LiTFSI at pH 1. The red and blue lines refer to samples immersed without and with Ar bubbling, respectively. The plots suggest that dissolved oxygen promotes p-type doping at lower pH by the uncontrolled way in contrast to BQ/HQ.
Extended Data Fig. 2 Measurements of electrode potentials during doping processes.
a, A setup for electrode potential measurements. Ag/AgCl and PBTTT electrodes were immersed in aqueous solutions with BQ/HQ and LiTFSI. The PBTTT electrode was fabricated by forming a PBTTT thin film on a Au-coated glass substrate. b, Doping measurement setup based on the in situ monitoring of the Fermi level of PBTTT. The doping level of the OSCs (not necessarily PBTTT), which varies depending on the redox potential of PCET-type agents, can be monitored using potential measurements and precisely controlled by the addition of an acid or base.
Extended Data Fig. 3 Maintained crystalline structures of PBTTT thin films in repeated oxidation and reduction processes.
a, b, Grazing-incidence wide-angle X-ray scattering images of a PBTTT thin film repeatedly immersed in aqueous solutions at pH 2 (a) and 4 (b), resulting in the oxidation and reduction, respectively, of the PBTTT film. The numbers above the images correspond to the cycle number shown in Fig. 4a in the main text. The similar diffraction patterns of (a) and (b) show no significant change in crystalline structure by successive immersion processes. c, d, XRD spectra of a PBTTT thin film repeatedly immersed in aqueous solutions at pH 2 and 4, whose conditions are same as (a) and (b), in the out-of-plane (c) and in-plane (d) directions.
Extended Data Fig. 4 Effective p-type doping over a wide range of pH values.
a-d, UV-vis-NIR spectra and the chemical structure of poly(3-hexylthiophene-2,5-diyl) (P3HT) (a–c) and conductivities of P3HT thin films immersed in aqueous solutions with BQ/HQ and LiNFSI at pH 1-7 (see Supplementary Information for details on the solution preparation). The error bars represent uncertainties in the conductivity due to uncertainties in the thickness of the P3HT thin films and represent one standard deviation. e, f, Summary of the d-spacing values calculated from the XRD results in the direction of out-of-plane (c) and in-plane (d). The error bars indicate uncertainties in the d-spacings due to compound errors resulting from the propagation of uncertainties in the fitting of the diffraction peaks and represent one standard deviation. All results show a trend similar to that of PBTTT (Fig. 2 in the main text), thus suggesting that our doping method is versatile and allows for the selection of materials to meet the desired purpose.
Extended Data Fig. 5 Evaluation of doping levels based on elemental analysis.
F/C atomic ratio of samples doped under various conditions (same conditions as that of Fig. 5 in the main text). The error bars represent uncertainties in the atomic ratio due to uncertainties in the fitting and represent one standard deviation. The horizontal dotted line corresponds to the doped state with one hole and one anion for the each monomer unit of polymer, which is calculated based on the atomic composition of PBTTT and dopant anions.
Supplementary information
Supplementary Information
Supplementary Methods and Discussion, including Supplementary Tables 1–4 and Figs 1–8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ishii, M., Yamashita, Y., Watanabe, S. et al. Doping of molecular semiconductors through proton-coupled electron transfer. Nature 622, 285–291 (2023). https://doi.org/10.1038/s41586-023-06504-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06504-8
This article is cited by
-
Dye-based nanoarchitectonics for the effective bandgap and stability of blue phosphorescent organic light-emitting diodes
Applied Physics A (2024)
-
Composite Nanoarchitectonics Towards Method for Everything in Materials Science
Journal of Inorganic and Organometallic Polymers and Materials (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.