Abstract
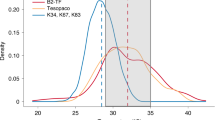
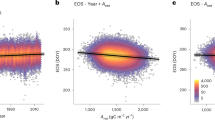
The critical temperature beyond which photosynthetic machinery in tropical trees begins to fail averages approximately 46.7 °C (Tcrit)1. However, it remains unclear whether leaf temperatures experienced by tropical vegetation approach this threshold or soon will under climate change. Here we found that pantropical canopy temperatures independently triangulated from individual leaf thermocouples, pyrgeometers and remote sensing (ECOSTRESS) have midday peak temperatures of approximately 34 °C during dry periods, with a long high-temperature tail that can exceed 40 °C. Leaf thermocouple data from multiple sites across the tropics suggest that even within pixels of moderate temperatures, upper canopy leaves exceed Tcrit 0.01% of the time. Furthermore, upper canopy leaf warming experiments (+2, 3 and 4 °C in Brazil, Puerto Rico and Australia, respectively) increased leaf temperatures non-linearly, with peak leaf temperatures exceeding Tcrit 1.3% of the time (11% for more than 43.5 °C, and 0.3% for more than 49.9 °C). Using an empirical model incorporating these dynamics (validated with warming experiment data), we found that tropical forests can withstand up to a 3.9 ± 0.5 °C increase in air temperatures before a potential tipping point in metabolic function, but remaining uncertainty in the plasticity and range of Tcrit in tropical trees and the effect of leaf death on tree death could drastically change this prediction. The 4.0 °C estimate is within the ‘worst-case scenario’ (representative concentration pathway (RCP) 8.5) of climate change predictions2 for tropical forests and therefore it is still within our power to decide (for example, by not taking the RCP 6.0 or 8.5 route) the fate of these critical realms of carbon, water and biodiversity3,4.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
We provide key data in the supplementary information. Data and code to produce all figures are available at https://doi.org/10.5061/dryad.fqz612jx1. Source data are provided with this paper.
Code availability
Data and code to produce all figures are available at https://doi.org/10.5061/dryad.fqz612jx1.
References
Slot, M. et al. Leaf heat tolerance of 147 tropical forest species varies with elevation and leaf functional traits, but not with phylogeny. Plant. Cell Environ. 44, 2414–2427 (2021).
IPCC. Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2013).
Wilson, E. & Raven, P. in Biodiversity (ed. Wilson, E. O.) Ch. 3 (National Academy Press, 1988).
Hubau, W. et al. Asynchronous carbon sink saturation in African and Amazonian tropical forests. Nature 579, 80–87 (2020).
Janzen, D. H. Why mountain passes are higher in the Tropics. Am. Nat. 101, 233–249 (1967).
Jiménez-Muñoz, J. C. et al. Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016. Sci. Rep. 6, 33130 (2016).
Jiménez-Muñoz, J. C., Sobrino, J. A., Mattar, C. & Malhi, Y. Spatial and temporal patterns of the recent warming of the Amazon forest. J. Geophys. Res. Atmos. 118, 5204–5215 (2013).
Doughty, C. E. & Goulden, M. L. Are tropical forests near a high temperature threshold? J. Geophys. Res. https://doi.org/10.1029/2007JG000632 (2008).
Sachs, J. Über die obere temperaturgränze der vegetation. Flora 47, 5–12 (1864).
Feeley, K. et al. The thermal tolerances, distributions, and performances of tropical montane tree species. Front. For. Glob. Change 3, 25 (2020).
Krause, G. H. et al. High-temperature tolerance of a tropical tree, Ficus insipida: methodological reassessment and climate change considerations. Funct. Plant Biol. 37, 890–900 (2010).
O’Sullivan, O. S. et al. Thermal limits of leaf metabolism across biomes. Glob. Chang. Biol. 23, 209–223 (2017).
Still, C. J. et al. Imaging canopy temperature: shedding (thermal) light on ecosystem processes. New Phytol. 230, 1746–1753 (2021).
Fisher, J. B. et al. ECOSTRESS: NASA’s next generation mission to measure evapotranspiration from the International Space Station. Water Resour. Res. 56, e2019WR026058 (2020).
Hulley, G. C. et al. Validation and quality assessment of the ECOSTRESS level-2 land surface temperature and emissivity product. IEEE Trans. Geosci. Remote Sens. 60, 1–23 (2022).
Fauset, S. et al. Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. Plant. Cell Environ. 41, 1618–1631 (2018).
Doughty, C. E. An in situ leaf and branch warming experiment in the Amazon. Biotropica 43, 658–665 (2011).
Carter, K. R., Wood, T. E., Reed, S. C., Butts, K. M. & Cavaleri, M. A. Experimental warming across a tropical forest canopy height gradient reveals minimal photosynthetic and respiratory acclimation. Plant. Cell Environ. 44, 2879–2897 (2021).
Rey-Sanchez, A. C., Slot, M., Posada, J. & Kitajima, K. Spatial and seasonal variation of leaf temperature within the canopy of a tropical forest. Clim. Res. 71, 75–89 (2016).
Crous, K. Y. et al. Similar patterns of leaf temperatures and thermal acclimation to warming in temperate and tropical tree canopies. Tree Physiol. tpad054 (2023).
Kivalov, S. N. & Fitzjarrald, D. R. Observing the whole-canopy short-term dynamic response to natural step changes in incident light: characteristics of tropical and temperate forests. Boundary Layer Meteorol. 173, 1–52 (2019).
Tiwari, R. et al. Photosynthetic quantum efficiency in south-eastern Amazonian trees may be already affected by climate change. Plant. Cell Environ. 44, 2428–2439 (2021).
da Costa, A. C. L. et al. Effect of 7 yr of experimental drought on vegetation dynamics and biomass storage of an eastern Amazonian rainforest. New Phytol. 187, 579–591 (2010).
Phillips, O. L. et al. Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347 (2009).
Gatti, L. V. et al. Amazonia as a carbon source linked to deforestation and climate change. Nature 595, 388–393 (2021).
Doughty, C. E. et al. Drought impact on forest carbon dynamics and fluxes in Amazonia. Nature 519, 78–82 (2015).
Hulley, G. C. & Hook, S. J. Generating consistent land surface temperature and emissivity products between ASTER and MODIS data for Earth science research. IEEE Trans. Geosci. Remote Sens. 49, 1304–1315 (2011).
Gillespie, A. et al. A temperature and emissivity separation algorithm for advanced spaceborne thermal emission and reflection radiometer (ASTER) images. IEEE Trans. Geosci. Remote Sens. 36, 1113–1126 (1998).
Kitudom, N. et al. Thermal safety margins of plant leaves across biomes under a heatwave. Sci. Total Environ. 806, 150416 (2022).
Berry, J. & Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31, 491–543 (1980).
Blonder, B. & Michaletz, S. T. A model for leaf temperature decoupling from air temperature. Agric. For. Meteorol. 262, 354–360 (2018).
Drake, J. E. et al. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Glob. Chang. Biol. 24, 2390–2402 (2018).
Guha, A. et al. Short-term warming does not affect intrinsic thermotolerance but induces strong sustaining photoprotection in tropical evergreen citrus genotypes. Plant. Cell Environ. 45, 105–120 (2022).
Smith, M. N. et al. Empirical evidence for resilience of tropical forest photosynthesis in a warmer world. Nat. Plants 6, 1225–1230 (2020).
Dickman, L. T. et al. Homoeostatic maintenance of nonstructural carbohydrates during the 2015–2016 El Niño drought across a tropical forest precipitation gradient. Plant. Cell Environ. 42, 1705–1714 (2019).
Subasinghe Achchige, Y. M., Volkova, L., Drinnan, A. & Weston, C. J. A quantitative test for heat-induced cell necrosis in vascular cambium and secondary phloem of Eucalyptus obliqua stems. J. Plant Ecol. 14, 160–169 (2021).
Tebaldi, C. et al. Climate model projections from the Scenario Model Intercomparison Project (ScenarioMIP) of CMIP6. Earth Syst. Dyn. 12, 253–293 (2021).
Rowland, L. et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 528, 119–122 (2015).
Vargas Zeppetello, L. R. et al. Large scale tropical deforestation drives extreme warming. Environ. Res. Lett. 15, 84012 (2020).
Araújo, I. et al. Trees at the Amazonia–Cerrado transition are approaching high temperature thresholds. Environ. Res. Lett. 16, 34047 (2021).
Miller, S. D. et al. Biometric and micrometeorological measurements of tropical forest carbon balance. Ecol. Appl. 14, 114–126 (2004).
da Rocha, H. R. et al. Seasonality of water and heat fluxes over a tropical forest in eastern Amazonia. Ecol. Appl. 14, 22–32 (2004).
Goulden, M. L. et al. Diel and seasonal patterns of tropical forest CO2 exchange. Ecol. Appl. 14, 42–54 (2004).
Jin, M. & Liang, S. An improved land surface emissivity parameter for land surface models using global remote sensing observations. J. Clim. 19, 2867–2881 (2006).
Miller, S. D. et al. Reduced impact logging minimally alters tropical rainforest carbon and energy exchange. Proc. Natl Acad. Sci. USA 108, 19431–19435 (2011).
Xiao, J., Fisher, J. B., Hashimoto, H., Ichii, K. & Parazoo, N. C. Emerging satellite observations for diurnal cycling of ecosystem processes. Nat. Plants 7, 877–887 (2021).
Kealy, P. S. & Hook, S. J. Separating temperature and emissivity in thermal infrared multispectral scanner data: implications for recovering land surface temperatures. IEEE Trans. Geosci. Remote Sens. 31, 1155–1164 (1993).
Reichle, R., De Lannoy, G., Koster, R. D., Crow, W. T. & Kimball, J. S. SMAP L4 9 km EASE-grid surface and root zone soil moisture geophysical data, version 3. National Snow and Ice Data Center https://doi.org/10.5067/B59DT1D5UMB4 (2017).
Slot, M., Krause, G. H., Krause, B., Hernández, G. G. & Winter, K. Photosynthetic heat tolerance of shade and sun leaves of three tropical tree species. Photosynth. Res. 141, 119–130 (2019).
Acknowledgements
Support was provided by the ECOSTRESS mission and NASA Research Opportunities in Space and Earth Science grant numbers 80NSSC20K0216, 80NSSC19K0206 and 80NSSC21K0191. S.F. and E.G. acknowledge Natural Environmental Research Council grant NE/V008366/1. K.C. acknowledges the Australian Research Council grant DE160101484.
Author information
Authors and Affiliations
Contributions
C.E.D., G.R.G., I.O.M., Y.M. and J.B.F. designed the study. C.E.D. and J.M.K. analysed the remote sensing data. C.E.D., M.L.G., H.R.d.R., S.D.M., S.F., E.G., C.R.-S., M.S., K.R.C., K.Y.C., K.B.M. and A.W.C. collected and analysed the empirical data. C.E.D. created the model. C.E.D. and B.C.W. prepared the public data and code. C.E.D. wrote the paper with contributions from G.R.G., K.Y.C., J.B.F. and I.O.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ben Bond-Lamberty, David Schimel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Regions of interest.
Tropical forest regions in A) Amazon, B) Central Africa and C) SE Asia used for the retrieval of ECOSTRESS LST and SMAP data. The red area was used to ground-truth ECOSTRESS LST with the pyrgeometer.
Extended Data Fig. 2 Impacts on canopy temperature.
(A) Linear regression of canopy temperature versus soil moisture (40 cm depth) at the km 83 eddy covariance tower (r2 = 0.46, P = 7e-10, N = 62). (B) Linear regression of canopy temperature as a function of air temperature during sunny periods during the wet (green circles) and dry (red circles) season at the km 83 eddy covariance tower in the Tapajos region of Brazil. Red line shows a linear fit for the dry season (r2 = 0.96, P = 3e-21, N = 29) and the lower line is a one-to-one line. (C) Linear regressions of canopy temperature as a function of latent heat flux for warm (>30 °C) periods (r2 = 0.50, P = 0.009, N = 11) at the km 83 eddy covariance tower in the Tapajos region of Brazil. (D) Linear regression (r2 = 0.75, P = 2e-5, N = 16) using data from Fig. 1a comparing ECOSTRESS dry season to pyrgeometer dry season data from the Tapajos (Km 83).
Extended Data Fig. 3 Histograms of canopy temperature.
Histograms of the canopy temperatures as (top) 30 min average periods and (bottom) two second instantaneous observations, where total shortwave energy load is >1000 W m−2, as measured by a downward facing pyrgeometer in the Tapajos region of Brazil.
Extended Data Fig. 4 Leaf thermocouple data from warming experiments.
Canopy top tropical leaf thermocouple measurements for normal (blue) and warmed leaves (red) for Brazil (+2 °C), Puerto Rico (+3 °C), and Australia (+4 °C). Insets show the long tail distribution of temperatures and text records the highest leaf temperature.
Extended Data Fig. 5 Leaf thermocouple data.
Canopy top tropical leaf thermocouple measurements for (top) Brazil km 67, (middle) Panama and (bottom) the Atlantic Forest in Brazil. Insets show the long tail distribution of temperatures and text records the highest leaf temperature. The resampled assumes a similar number of samples (~N = 400) at 38 °C for both sites and fits a curve to extrapolate the long tail. The Atlantic forest is a cooler forest (at ~1000 m) and the median temperature of the Amazon is ~4 °C higher than the Atlantic forest.
Extended Data Fig. 6 Duration of warming.
Periods when the leaves were warmed by >8 min during the Tapajos warming experiment for individual leaves (thin lines) and averaged (thick red line). Text in figure indicates the percent of time leaves exceeded Tcrit for greater than 6 and 8 min.
Extended Data Fig. 7 Finding African peak temperatures.
Procedure for finding peak canopy temperatures using ECOSTRESS data for central Africa. (A) Log10 histogram of temperatures for (B) a region of Central Africa. A diurnal curve showing all ECOSTRESS LST data for central Africa versus (C) time of day and (D) time of year. (E) SMAP soil moisture (m3 m−3) data showing periods of dry weather.
Extended Data Fig. 8 Finding SE Asian peak temperatures.
Procedure for finding peak canopy temperatures using ECOSTRESS data for SE Asia. (A) Log10 histogram of temperatures for (B) a region of Central Africa. A diurnal curve showing all ECOSTRESS LST data for SE Asia versus (C) time of day and (D) time of year. (E) SMAP soil moisture data (m3 m−3) showing periods of dry weather.
Extended Data Fig. 9 Comparison of LST temperature data.
We show the spatial distribution of LST data for three sensors (VIIRS, MODIS, and ECOSTRESS) for similar time periods (Sept 18–28, 2019) for similar areas in the Amazon basin. The difference between the left, middle and right are different data quality flags for no flag (left), QF g1 from Supplementary Table 1 (middle) and QF g2 (right). We used three levels of quality flags (ECOSTRESS – G1 - 3522 and 3520, G2 =3520, VIIRS – G1 – 12001, 15841, 11745, 32225 and G2 = 32225, and MODIS – G1 - 0 and 65 and G2 -0) for the region depicted in Extended Data Fig. 1a during the same period (18 September to 28 September 2019). Quality flags were complex with 136 for ECOSTRESS and 229 for VIIRS (but only 8 for MODIS).
Extended Data Fig. 10 Histogram of LST temperature data.
(top) We show log10 histograms of LST data for three sensors (VIIRS, MODIS, and ECOSTRESS) for similar time periods (Sept 18–28, 2019) for similar areas in the Amazon basin. The difference between the left, middle and right are different data quality flags for no flag (left), QF g1 from Supplementary Table 1 (middle) and QF g2 (right). We used three levels of quality flags (ECOSTRESS – G1 - 3522 and 3520, G2 =3520, VIIRS – G1 – 12001, 15841, 11745, 32225 and G2 = 32225, and MODIS – G1 - 0 and 65 and G2 -0) for the region depicted in Extended Data Fig. 1a during the same period (18 September to 28 September 2019). (bottom) - A scaled in comparison for the same dataset showing the much higher resolution of ECOSTRESS versus VIIRS and MODIS LST.
Supplementary information
Supplementary Information
This file contains supplementary text, methods and Tables 1, 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doughty, C.E., Keany, J.M., Wiebe, B.C. et al. Tropical forests are approaching critical temperature thresholds. Nature 621, 105–111 (2023). https://doi.org/10.1038/s41586-023-06391-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06391-z
This article is cited by
-
Critical transitions in the Amazon forest system
Nature (2024)
-
Synthesis of the land carbon fluxes of the Amazon region between 2010 and 2020
Communications Earth & Environment (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.