Abstract
Biomolecular condensates formed by phase separation can compartmentalize and regulate cellular processes1,2. Emerging evidence has suggested that membraneless subcellular compartments in virus-infected cells form by phase separation3,4,5,6,7,8. Although linked to several viral processes3,4,5,9,10, evidence that phase separation contributes functionally to the assembly of progeny particles in infected cells is lacking. Here we show that phase separation of the human adenovirus 52-kDa protein has a critical role in the coordinated assembly of infectious progeny particles. We demonstrate that the 52-kDa protein is essential for the organization of viral structural proteins into biomolecular condensates. This organization regulates viral assembly such that capsid assembly is coordinated with the provision of viral genomes needed to produce complete packaged particles. We show that this function is governed by the molecular grammar of an intrinsically disordered region of the 52-kDa protein, and that failure to form condensates or to recruit viral factors that are critical for assembly results in failed packaging and assembly of only non-infectious particles. Our findings identify essential requirements for coordinated assembly of progeny particles and demonstrate that phase separation of a viral protein is critical for production of infectious progeny during adenovirus infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All graphed numerical data are available as source data. Uncropped western blots and Coomassie gels are included in Supplementary Fig. 1. Unprocessed images are available on request from the corresponding authors. Source data are provided with this paper.
Change history
23 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41586-023-06343-7
References
Hyman, A. A., Weber, C. A. & Jülicher, F. Liquid–liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Etibor, T. A., Yamauchi, Y. & Amorim, M. J. Liquid biomolecular condensates and viral lifecycles: review and perspectives. Viruses 13, 366 (2021).
Su, J. M., Wilson, M. Z., Samuel, C. E. & Ma, D. Formation and function of liquid-like viral factories in negative-sense single-stranded RNA virus infections. Viruses 13, 126 (2021).
Sengupta, P. & Lippincott-Schwartz, J. Revisiting membrane microdomains and phase separation: a viral perspective. Viruses 12, 745 (2020).
Risso-Ballester, J. et al. A condensate-hardening drug blocks RSV replication in vivo. Nature 595, 596–599 (2021).
Heinrich, B. S., Maliga, Z., Stein, D. A., Hyman, A. A. & Whelan, S. P. J. Phase transitions drive the formation of vesicular stomatitis virus replication compartments. mBio 9, e02290-17 (2018).
Hidalgo, P. et al. Evidence that the adenovirus single-stranded DNA binding protein mediates the assembly of biomolecular condensates to form viral replication compartments. Viruses 13, 1778 (2021).
Iserman, C. et al. Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol. Cell 80, 1078–1091.e6 (2020).
Guseva, S. et al. Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci. Adv. 6, eaaz7095 (2020).
Li, H. et al. Phase separation in viral infections. Trends Microbiol. 30, 1217–1231 (2022).
Charman, M. & Weitzman, M. D. Replication compartments of DNA viruses in the nucleus: location, location, location. Viruses 12, 151 (2020).
Charman, M., Herrmann, C. & Weitzman, M. D. Viral and cellular interactions during adenovirus DNA replication. FEBS Lett. 593, 3531–3550 (2019).
Ahi, Y. S. & Mittal, S. K. Components of adenovirus genome packaging. Front. Microbiol. 7, 1503 (2016).
Condezo, G. N. et al. Structures of adenovirus incomplete particles clarify capsid architecture and show maturation changes of packaging protein L1 52/55k. J. Virol. 89, 9653–9664 (2015).
Gustin, K. E. & Imperiale, M. J. Encapsidation of viral DNA requires the adenovirus L1 52/55-kilodalton protein. J. Virol. 72, 7860–7870 (1998).
Hasson, T. B., Soloway, P. D., Ornelles, D. A., Doerfler, W. & Shenk, T. Adenovirus L1 52- and 55-kilodalton proteins are required for assembly of virions. J. Virol. 63, 3612–3621 (1989).
Russell, W. C. & Skehel, J. J. The polypeptides of adenovirus-infected cells. J. Gen. Virol. 15, 45–57 (1972).
Pombo, A., Ferreira, J., Bridge, E. & Carmo-Fonseca, M. Adenovirus replication and transcription sites are spatially separated in the nucleus of infected cells. EMBO J. 13, 5075–5085 (1994).
Pied, N. & Wodrich, H. Imaging the adenovirus infection cycle. FEBS Lett. 593, 3419–3448 (2019).
Su Hui Teo, C., Serwa, R. A. & O’Hare, P. Spatial and temporal resolution of global protein synthesis during HSV infection using bioorthogonal precursors and click chemistry. PLoS Pathog. 12, e1005927 (2016).
Livingston, C. M., Ifrim, M. F., Cowan, A. E. & Weller, S. K. Virus-induced chaperone-enriched (VICE) domains function as nuclear protein quality control centers during HSV-1 infection. PLoS Pathog. 5, e1000619 (2009).
Brangwynne, C. P., Tompa, P. & Pappu, R. V. Polymer physics of intracellular phase transitions. Nat. Phys. 11, 899–904 (2015).
Holehouse, A. S. & Pappu, R. V. Functional implications of intracellular phase transitions. Biochemistry 57, 2415–2423 (2018).
Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012).
Kroschwald, S., Maharana, S. & Simon, A. Hexanediol: a chemical probe to investigate the material properties of membrane-less compartments. Matters 3, e201702000010 (2017).
Alberti, S., Gladfelter, A. & Mittag, T. Considerations and challenges in studying liquid–liquid phase separation and biomolecular condensates. Cell 176, 419–434 (2019).
Alberti, S. et al. A user’s guide for phase separation assays with purified proteins. J. Mol. Biol. 430, 4806–4820 (2018).
Ma, H.-C. & Hearing, P. Adenovirus structural protein IIIa is involved in the serotype specificity of viral DNA packaging. J. Virol. 85, 7849–7855 (2011).
Wang, J. et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699.e16 (2018).
Gomes, E. & Shorter, J. The molecular language of membraneless organelles. J. Biol. Chem. 294, 7115–7127 (2019).
Chong, S. & Mir, M. Towards decoding the sequence-based grammar governing the functions of intrinsically disordered protein regions. J. Mol. Biol. 433, 166724 (2021).
Greig, J. A. et al. Arginine-enriched mixed-charge domains provide cohesion for nuclear speckle condensation. Mol. Cell 77, 1237–1250.e4 (2020).
Lin, Y.-H., Brady, J. P., Forman-Kay, J. D. & Chan, H. S. Charge pattern matching as a ‘fuzzy’ mode of molecular recognition for the functional phase separations of intrinsically disordered proteins. New J. Phys. 19, 115003 (2017).
Nott, T. J. et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57, 936–947 (2015).
Pfitzner, S. et al. Fluorescent protein tagging of adenoviral proteins pV and pIX reveals ‘late virion accumulation compartment’. PLoS Pathog. 16, e1008588 (2020).
Condezo, G. N. & San Martín, C. Localization of adenovirus morphogenesis players, together with visualization of assembly intermediates and failed products, favor a model where assembly and packaging occur concurrently at the periphery of the replication center. PLoS Pathog. 13, e1006320 (2017).
Gustin, K. E., Lutz, P. & Imperiale, M. J. Interaction of the adenovirus L1 52/55-kilodalton protein with the IVa2 gene product during infection. J. Virol. 70, 6463–6467 (1996).
Perlmutter, J. D. & Hagan, M. F. Mechanisms of virus assembly. Annu. Rev. Phys. Chem. 66, 217–239 (2015).
Katen, S. & Zlotnick, A. The thermodynamics of virus capsid assembly. Methods Enzymol. 455, 395–417 (2009).
Zacharias, D. A., Violin, J. D., Newton, A. C. & Tsien, R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002).
Busnadiego, I. et al. Host and viral determinants of Mx2 antiretroviral activity. J. Virol. 88, 7738–7752 (2014).
Kozarsky, K. F., Jooss, K., Donahee, M., Strauss, J. F. & Wilson, J. M. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat. Genet. 13, 54–62 (1996).
Hermann, C. et al. Adenovirus-mediated ubiquitination alters protein–RNA binding and aids viral RNA processing. Nat. Microbiol. 5, 1217–1231 (2020).
Ostapchuk, P., Yang, J., Auffarth, E. & Hearing, P. Functional interaction of the adenovirus IVa2 protein with adenovirus type 5 packaging sequences. J. Virol. 79, 2831–2838 (2005).
Yan, J. et al. Interaction between hexon and L4-100K determines virus rescue and growth of hexon-chimeric recombinant Ad5 vectors. Sci. Rep. 6, 22464 (2016).
Wu, K., Guimet, D. & Hearing, P. The adenovirus L4-33K protein regulates both late gene expression patterns and viral DNA packaging. J. Virol. 87, 6739–6747 (2013).
Reich, N. C., Sarnow, P., Duprey, E. & Levine, A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology 128, 480–484 (1983).
Sarnow, P., Sullivan, C. A. & Levine, A. J. A monoclonal antibody detecting the adenovirus type 5-E1b-58Kd tumor antigen: characterization of the E1b-58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology 120, 510–517 (1982).
Marton, M. J., Baim, S. B., Ornelles, D. A. & Shenk, T. The adenovirus E4 17-kilodalton protein complexes with the cellular transcription factor E2F, altering its DNA-binding properties and stimulating E1A-independent accumulation of E2 mRNA. J. Virol. 64, 2345–2359 (1990).
Price, A. M. et al. Novel viral splicing events and open reading frames revealed by long-read direct RNA sequencing of adenovirus transcripts. PLoS Pathog. 18, e1010797 (2022).
Johnson, J. S. et al. Adenovirus protein VII condenses DNA, represses transcription, and associates with transcriptional activator E1A. J. Virol. 78, 6459–6468 (2004).
Komatsu, T., Dacheux, D., Kreppel, F., Nagata, K. & Wodrich, H. A method for visualization of incoming adenovirus chromatin complexes in fixed and living cells. PLoS ONE 10, e0137102 (2015).
Price, A. M. et al. Direct RNA sequencing reveals m6A modifications on adenovirus RNA are necessary for efficient splicing. Nat. Commun. 11, 6016 (2020).
Mészáros, B., Erdos, G. & Dosztányi, Z. IUPred2A: context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 46, W329–W337 (2018).
Lancaster, A. K., Nutter-Upham, A., Lindquist, S. & King, O. D. PLAAC: a web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 30, 2501–2502 (2014).
Holehouse, A. S., Das, R. K., Ahad, J. N., Richardson, M. O. G. & Pappu, R. V. CIDER: resources to analyze sequence–ensemble relationships of intrinsically disordered proteins. Biophys. J. 112, 16–21 (2017).
van Mierlo, G. et al. Predicting protein condensate formation using machine learning. Cell Rep. 34, 108705 (2021).
Acknowledgements
We thank members of the Weitzman laboratory for insightful discussions and input; P. Hearing, M. Imperiale, C. Boutell, A. Levine, D. Ornelles and J. Wilson for gifts of reagents; J. Burkhardt for the use of the live-cell microscope; B. Portz, J. Shorter, L. Wan, M. A. Mir, J. B. Weitzman, K. F. Liu and D. Bracha for their thoughtful feedback and careful reading of the manuscript; and the UPenn Cell and Developmental Biology Microscopy Core for imaging assistance. This research was supported by NIAID grants R01-AI145266 and R01-AI121321 (to M.D.W.). M.D.W. was partially supported by NCI grant P30-CA016520. N.G. was partially supported by NIGMS grant T32-GM007229.
Author information
Authors and Affiliations
Contributions
M.C. conceived and led the project, contributed to experimental design, the execution of experiments (including confocal and live-cell microscopy, virology and cell biology experiments, and in vitro biochemistry) and analysis of data, and led preparation and writing of the manuscript. N.G. contributed to experimental design, the execution of experiments (including virology and cell biology experiments, and propagation of mutant virus pm8001) and data analysis. N.K. contributed to the confocal and live-cell imaging experiments. E.H. and K.K.L. contributed to western blotting. A.A. contributed to the execution of experiments (including in vitro biochemistry and microscopy). J.M.D. contributed to bioinformatics data. D.B. contributed to FRAP and live-cell microscopy experiments. E.T. contributed to preliminary experiments and blinded phenotyping analysis. M.D.W. obtained funding, supervised all research and contributed to writing the manuscript. All authors contributed to preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Carmen San Martín and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
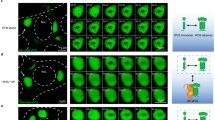
Extended Data Fig. 1 Localization of viral proteins in the adenovirus infected nucleus.
Uninfected or infected (AdV) human bronchial epithelial cells as indicated (hours post-infection, hpi). a, Localization of indicated viral proteins in uninfected or WT adenovirus infected (AdV) human bronchial epithelial cells at 22 hpi. b, Localization of HPG-labeled protein synthesized within 30 min prior to fixation. Viral nuclear bodies and viral replication compartments are marked by immunostaining of 52K or DBP, respectively. Zoom shows magnified 10 x 10 μm area corresponding to dotted white box. Line profile corresponding to dotted black line indicates lack of co-localization between 52K and DBP. c, Localization of 52K to viral nuclear bodies in cells infected at a multiplicity of infection (MOI) of 0.01, 0.1, 1, or 10 plaque forming units per cell. d, Localization of heat shock cognate 70 (Hsc70) in relation to 52K. All scale bars = 10 µm, outlines of nuclei (doted white lines) are shown (a,c,d).
Extended Data Fig. 2 Additional characterization of viral nuclear bodies.
a, Wide view immunofluorescence images of nuclear bodies (52K) and viral replication compartments (DBP) in WT adenovirus infected (AdV) human bronchial epithelial cells incubated with or without 10% 1,6-hexanediol corresponding to single nuclei images presented in Fig. 1g. b–h, Uninfected or WT adenovirus infected doxycycline-inducible transgenic A549 lung cells. b, Expression of transgenes (GFP, 52K-GFP) with or without induction by addition of doxycycline (DOX) in relation to endogenous 52K. GAPDH is included as a sample processing control. c, Localization of ectopically expressed 52K-GFP to nuclear bodies shown by co-staining of endogenous IIIa. d, Localization of ectopically expressed GFP and endogenous IIIa. e, Half time of fluorescence recovery of nuclear diffuse 52K-GFP in uninfected cells corresponding to (f), or nuclear bodies in WT adenovirus infected cells corresponding to Fig. 1h,i. Three independent repeats pooled with a total of 15 (uninfected) or 22 (nuclear bodies) bleached regions analyzed. Mean (columns) and standard deviation (error bars) shown. f, Fluorescence recovery after photobleaching of nuclear diffuse 52K-GFP in uninfected cells. Three independent repeats pooled with replicates representing 15 bleached regions (green lines) and mean (black line) shown. g, % fluorescence recovery of nuclear diffuse 52K-GFP in uninfected cells corresponding to (f), or nuclear bodies in WT adenovirus infected cells corresponding to Fig. 1h,i. Three independent repeats pooled with a total of 15 (uninfected) or 22 (nuclear bodies) bleached regions analyzed. Mean (columns) and standard deviation (error bars) shown. h, Fusion events observed per cell. Eight cells were imaged for five minutes. Image scale bars = 10 μm, outlines of nuclei (doted white lines) shown (a,c,d). Unpaired two-sided Student’s t-test (e,g), ns p > 0.05, **** p < 0.0001. Additional statistics including exact P values are included in Supplementary Notes. Gel source data are included in Supplementary Fig. 1.
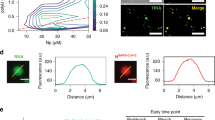
Extended Data Fig. 3 Supporting data corresponding to in vitro phase-separation of the 52K protein.
a, SDS-PAGE and Coomassie staining of protein showing cleavage of the MBP tag from WT 52K, or IDR mutants (Δ1-119, R/K, Scramble, P/A, Q/G) by addition of tobacco etch virus (TEV) protease at a ratio of 1:25 and incubation at 25 °C for 1 h. b, In vitro phase-separation of WT 52K or IDR mutants at the indicated protein concentrations. c, SDS-PAGE and Coomassie staining of protein showing cleavage of MBP tag from IIIa by addition of TEV protease at a ratio of 1:25 and incubation at 25 °C for 1 h. d, In vitro phase-separation of IIIa at indicated concentrations. All image scale bars = 10 μm. Gel source data are included in Supplementary Fig. 1.
Extended Data Fig. 4 Additional characterization of the Δ52K mutant adenovirus.
a, CsCl gradient purification of adenovirus particles from WT or Δ52K adenovirus infected 293 cells. Incomplete and packaged particles are indicated. b, Examples of diffuse, nuclear body, and peripheral localization of IIIa in human bronchial epithelial cells infected with WT or Δ52K adenovirus. Outlines of nuclei (dotted white lines) are shown, image scale bar = 10 μm. c, Quantification (%) of WT or Δ52K adenovirus infected human bronchial epithelial cells corresponding to each localization phenotype presented in (b). Three independent repeats plotted, with mean (columns) and standard deviation (error bars) shown. Unpaired two-sided Welch’s t-tests (c). ns p > 0.05, * p < 0.05, *** p < 0.001. Additional statistics including exact P values are included in Supplementary Notes.
Extended Data Fig. 5 In silico analysis of the 52K protein.
a–c, analysis of amino acid composition of the 52K protein. a, Predictions of fold-index, hydrophobicity, fraction of charged residues (FCR), and net charge per residue (NCPR). b, Amino acid frequency corresponding to full length 52K or the N-terminal 145 amino acids compared to the mean frequency of proteins in the human proteome (Human; n = 20371 proteins) or protein phase separation database (PPS; n = 90 proteins). Mean (columns) and standard deviation (error bars) are shown. c, Amino acid frequency of the N-terminal region (1-145) compared to the C-terminal region (146-415). d, Net charge per residue (NCPR) comparing the N-terminal region 1-145 to the corresponding region of the scramble mutant. e, Alignment corresponding to amino acids 1-145 of WT 52K or IDR mutants (R/K, Scramble, P/A, Q/G). Mutated amino acids are highlighted.
Extended Data Fig. 6 Supporting data corresponding to fluorescence recovery after photobleaching of ectopic nuclear bodies.
HEK 293T cells transiently expressing GFP-tagged WT or mutant (P/A, Q/G) 52K, corresponding to the experiment presented in Fig. 3g–i. a, Western blots showing transient expression. GAPDH is included as a sample processing control. b, Examples of fluorescence recovery after photobleaching, prior to photobleaching (pre-bleach), immediately after photobleaching (bleach) or following recovery (post-bleach). Targeted nuclear bodies are indicated by yellow arrows. Gel source data including additional loading controls are included in Supplementary Fig. 1.
Extended Data Fig. 7 Supporting data corresponding to late-phase reorganization of the adenovirus-infected nucleus.
a-f, Uninfected or WT adenovirus infected (AdV) human bronchial epithelial cells as indicated (hours post-infection, hpi). a, Maximum intensity projections of nuclei stained by DAPI over a time-course of virus infection spanning 0-34 hpi. b, Nucleus size corresponding to (a). The total number of nuclei analyzed were 187 (Uninfected or 22 hpi), 179 (16 hpi), 157 (28 hpi), or 143 (34 hpi). c, Visualization of nuclear reorganization by immunostaining of laminA. Line profile corresponding to dashed yellow line is shown. d, Visualization of nuclear reorganization by immunostaining of histone H1. Line profile corresponding to dashed yellow line is shown. e, Changes in nucleus and viral replication compartment morphology over a time-course of virus infection (16-28 hpi) corresponding to Fig. 4a, shown by DAPI staining and immunostaining of DBP. f, Localization of 52K at 34 hpi. DNA is stained with DAPI. Examples of the late virion accumulation compartments identified by DAPI staining and the absence of 52K staining are indicated (yellow arrows). Zoom shows magnified 35 x 35 μm area corresponding to dotted white box. g, Live imaging of 52K-GFP in WT adenovirus infected transgenic A549 lung cells showing the progression of 52K-localization - from nuclear bodies to peripheral. Subsequent panels represent 15-minute intervals. h, Purified dsDNA corresponding to the 36 kbp AdV genome (vDNA). Digestion of DNA by Benzonase is included for quality control. i, Confocal images of in vitro phase-separation assays using 10 μM 488-labelled 52K (green) and indicated concentrations of vDNA (0-3200 pM). j, Number of condensates detected by analyzing a total of nine fields of view (three full repeats comprising three technical repeats each). k, Condensate size corresponding to (i). Median (line), interquartile interval (box), and 5-95 percentiles (whiskers) are shown. The total number of condensates analyzed were 3164 (0 pM), 9688 (200 pM), 16207 (400 pM), 14839 (800 pM), 800 (1600 (pM), or 330 (3200 pM). l, In vitro phase-separation of 52K at a concentration of 10 μM without (− vDNA), or with untreated (− Benzonase) or benzonase treated (+ Benzonase) viral DNA (+ vDNA) at a concentration of 800 pM. All image scale bars = 10 µm. Three independent repeats pooled (b,k) or plotted (j), with mean (columns or line) and standard deviation (error bars) shown (b,j). Kruskal-Wallis with Dunn’s tests (b,k) or one-way ANOVA with Šídák’s tests (j). ns p > 0.05, ** p < 0.01, **** p < 0.0001. Additional statistics including exact P values are included in Supplementary Notes. Gel source data are included in Supplementary Fig. 1.
Extended Data Fig. 8 Additional characterization of the temperature-sensitive mutant adenovirus.
a, SDS-PAGE of viral particles purified from WT, Δ52K, or temperature sensitive (TS) adenovirus infected 293 cells incubated at 39.5 °C. Packaged (P) and incomplete (I) particles are indicated. Hexon, penton, and fiber were detected by Coomassie staining. IIIa, 52K, and the unprocessed (pre-VII) and processed (VII) forms of protein VII were detected by immunoblot blot. b, Localization of 52K and viral replication compartments (DBP) in human bronchial epithelial cells infected with WT or temperature sensitive (TS) adenovirus and incubated at the non-permissive temperature of 39.5 °C. c, Wide view images of 52K localization in human bronchial epithelial cells infected with WT or temperature sensitive (TS) adenovirus and incubated at the non-permissive temperature of 39.5 °C. d, Localization of WT or temperature sensitive (TS) 52K-GFP transient expressed in 293T cells incubated at the non-permissive temperature of 39.5 °C. DNA is stained with DAPI. e, Western blots showing accumulation of viral late proteins in human bronchial epithelial cells infected with WT or temperature sensitive (TS) adenovirus and incubated at the permissive temperature of 32.0 °C. GAPDH is included as a sample processing control. f, Localization of 52K or IIIa human bronchial epithelial cells infected with WT or temperature sensitive (TS) adenovirus and incubated at the permissive temperature of 32.0 °C. Viral replication compartments marked by immunostaining of the viral DNA-binding protein (DBP) are shown. All image scale bars = 10 μm, outlines of nuclei (dotted white lines) are shown (b,c,f). Gel source data including additional loading controls are included in Supplementary Fig. 1.
Extended Data Fig. 9 Supporting data corresponding to complementation of the Δ52K mutant adenovirus.
a,b, Transient expression of WT or mutant (Q/G, P/A, R/K, Scramble) in 293T cells infected with Δ52K adenovirus. Cells that express no transgene act as a negative control. a, Immunoprecipitation of WT or mutant 52K. Input (1%) is shown. b, Complementation of progeny production. Progeny production in WT virus infected cells without trans gene expression is shown for context. c,d, transgenic A549 lung cells expressing R/K or matched WT 52K control (Set 1) or P/A and matched WT 52K control (Set 2). c, Localization of 52K. d, % of cells with nuclear bodies. e, SDS-PAGE and Coomassie staining of protein showing cleavage of the MBP tag from 52K mutant Δ1-119 by addition of TEV protease at a ratio of 1:50 and incubation at 25 °C for 1 h. f, In vitro phase-separation of 52K mutant Δ1-119 at the indicated protein concentrations. g–j, Parental A549 lung cells or transgenic A549 lung cells expressing WT 52K or Δ1-47, uninfected (Un) or infected with WT or Δ52K adenovirus as indicated. g, Accumulation of viral late proteins with GAPDH as loading control. h, Localization of WT 52K or Δ1-47 in relation to viral replication compartments (DBP). i, Complementation of progeny production in Δ52K infected cells. Progeny production in WT adenovirus infected parental control cells is included for context. j, Incomplete or packaged particles isolated by CsCl gradient purification. All image scale bars = 10 μm, outlines of nuclei (dotted white lines) are shown (c,h). Three independent repeats plotted (b,d,i), with mean (columns) and standard deviation (error bars) shown. ANOVA with Dunnett’s (b), Šídák’s (d), or Tukey’s tests (i). ns p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Additional statistics including exact P values are included in Supplementary Notes. Gel source data including additional loading controls are included in Supplementary Fig. 1.
Extended Data Fig. 10 Proposed model of adenovirus assembly.
The 52K protein and capsid proteins phase-separate, concentrating these proteins within biomolecular condensates, while limiting their concentration in the nucleoplasm. Incomplete, empty capsids assemble from viral proteins in the nucleoplasm. The organization of viral proteins into biomolecular condensates is a pre-requisite for assembly of complete packaged particles, which is mediated by the provision of viral genomes and their associated core and packaging proteins. Top panel: Mutation of arginine to lysine (R/K) within the intrinsically disordered region of 52K protein prevents phase-separation. Mutation of proline to alanine (P/A) in the intrinsically disordered spacer region compromises coordinated assembly downstream of biomolecular condensates. In either case this results in the assembly of only incomplete particles. Bottom panel: Biomolecular condensates formed during infection with ts369 at the non-permissive temperature of 39.5 °C do not concentrate IIIa, a key determinant of successful packaging. This results in failed packaging, and assembly of only incomplete particles.
Supplementary information
Supplementary Figure 1
Gel source data including uncropped images of western blots and Coomassie stained gels, and additional gel loading and sample processing controls not shown in Figures or Extended Data.
Supplementary Notes
Additional notes and information on sample size and statistics, including test statistics, confidence intervals, effect sizes, degrees of freedom and exact P values.
Supplementary Video 1
Fusion of Viral Nuclear Bodies. Live cell imaging showing the fusion of viral nuclear bodies in cells infected with WT adenovirus. Viral biomolecular condensates are visualized by the recruitment of 52K-GFP expressed in trans. Live cell imaging was captured at a frame rate of 1 frame per second and is presented as a video with a frame rate of 10 frames per second, representing a 5-minute imaging period. The video is displayed using the Fire lookup table from FIJI to highlight sub-compartments enriched for 52K-GFP.
Supplementary Video 2
Progression of 52K Localization Phenotype. Live cell imaging showing the progression of 52K localization phenotype from punctate nuclear bodies to peripheral accumulates in cells infected with WT adenovirus. Viral biomolecular condensates are visualized by the recruitment of 52K-GFP expressed in trans. Live cell imaging was captured at a frame rate of 20 frames per hour and is presented as a video with a frame rate of 4 frames per second, representing a 3-hour imaging period.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Charman, M., Grams, N., Kumar, N. et al. A viral biomolecular condensate coordinates assembly of progeny particles. Nature 616, 332–338 (2023). https://doi.org/10.1038/s41586-023-05887-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05887-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.