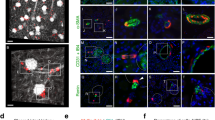
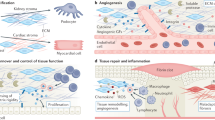
Abstract
Perivascular niches in the kidney comprise heterogeneous cell populations, including pericytes and fibroblasts, with distinct functions. These perivascular cells have crucial roles in preserving kidney homeostasis as they maintain microvascular networks by stabilizing the vasculature and regulating capillary constriction. A subset of kidney perivascular cells can also produce and secrete erythropoietin; this ability can be enhanced with hypoxia-inducible factor-prolyl hydroxylase inhibitors, which are used to treat anaemia in chronic kidney disease. In the pathophysiological state, kidney perivascular cells contribute to the progression of kidney fibrosis, partly via transdifferentiation into myofibroblasts. Moreover, perivascular cells are now recognized as major innate immune sentinels in the kidney that produce pro-inflammatory cytokines and chemokines following injury. These mediators promote immune cell infiltration, leading to persistent inflammation and progression of kidney fibrosis. The crosstalk between perivascular cells and tubular epithelial, immune and endothelial cells is therefore a key process in physiological and pathophysiological states. Here, we examine the multiple roles of kidney perivascular cells in health and disease, focusing on the latest advances in this field of research.
Key points
-
Perivascular niches in the kidney comprise heterogeneous cell populations, including pericytes and fibroblasts, with distinct functions. These perivascular cells have crucial roles in maintaining homeostasis in the kidney.
-
Pericytes maintain microvascular networks by stabilizing the vasculature and modulating the constriction of capillaries. In pathological states, such as ischaemia–reperfusion, pericytes contribute to the ‘no-reflow’ phenomenon.
-
A subset of kidney perivascular cells can produce and secrete erythropoietin; hypoxia-inducible factor-prolyl hydroxylase inhibitors can boost this secretion and are used for the treatment of anaemia in chronic kidney disease.
-
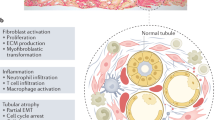
Perivascular cells or platelet-derived growth factor receptor-β+ (PDGFRβ+) pericytes synthesize and secrete various intracellular complement proteins and contribute to expression of collagen and extracellular matrix observed during the development of kidney fibrosis.
-
In pathophysiological states, kidney perivascular cells contribute to the progression of kidney fibrosis, partly by transdifferentiating into myofibroblasts.
-
Perivascular cells are major innate immune sentinels in the kidney and produce pro-inflammatory cytokines and chemokines after injury; these mediators promote immune cell infiltration, leading to persistent inflammation and progression of kidney fibrosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic kidney disease. Lancet 389, 1238–1252 (2017).
Eckardt, K. U. et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382, 158–169 (2013).
Keith, D. S., Nichols, G. A., Gullion, C. M., Brown, J. B. & Smith, D. H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 164, 659–663 (2004).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
National Kidney, F. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am. J. Kidney Dis. 60, 850–886 (2012).
United States Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Digestive and Kidney Diseases, Vol. 2014 (Bethesda, 2013).
Souma, T. et al. Plasticity of renal erythropoietin-producing cells governs fibrosis. J. Am. Soc. Nephrol. 24, 1599–1616 (2013).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Asada, N. et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J. Clin. Invest. 121, 3981–3990 (2011).
Lin, S. L., Kisseleva, T., Brenner, D. A. & Duffield, J. S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Leaf, I. A. et al. Pericyte MyD88 and IRAK4 control inflammatory and fibrotic responses to tissue injury. J. Clin. Invest. 127, 321–334 (2017).
Stark, K. et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat. Immunol. 14, 41–51 (2013).
Kaneko, K. et al. Lineage tracing analysis defines erythropoietin-producing cells as a distinct subpopulation of resident fibroblasts with unique behaviors. Kidney Int. 102, 280–292 (2022).
Broeker, K. A. E. et al. Different subpopulations of kidney interstitial cells produce erythropoietin and factors supporting tissue oxygenation in response to hypoxia in vivo. Kidney Int. 98, 918–931 (2020).
Chang, Y. T. et al. DNA methyltransferase inhibition restores erythropoietin production in fibrotic murine kidneys. J. Clin. Invest. 126, 721–731 (2016).
Shaw, I., Rider, S., Mullins, J., Hughes, J. & Peault, B. Pericytes in the renal vasculature: roles in health and disease. Nat. Rev. Nephrol. 14, 521–534 (2018).
Lin, A. et al. Mural cells: potential therapeutic targets to bridge cardiovascular disease and neurodegeneration. Cells 10, 593 (2021).
Boyle, S. C., Liu, Z. & Kopan, R. Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development 141, 346–354 (2014).
Lemos, D. R. et al. Maintenance of vascular integrity by pericytes is essential for normal kidney function. Am. J. Physiol. Renal Physiol. 311, F1230–F1242 (2016).
Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Sims, D. E. The pericyte — a review. Tissue Cell 18, 153–174 (1986).
Kennedy-Lydon, T. M., Crawford, C., Wildman, S. S. & Peppiatt-Wildman, C. M. Renal pericytes: regulators of medullary blood flow. Acta Physiol. 207, 212–225 (2013).
Teichert, M. et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 8, 16106 (2017).
Payne, L. B. et al. The pericyte microenvironment during vascular development. Microcirculation 26, e12554 (2019).
Kramann, R. et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015).
Murray, I. R. et al. αv integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat. Commun. 8, 1118 (2017).
Volz, K. S. et al. Pericytes are progenitors for coronary artery smooth muscle. eLife 4, e10036 (2015).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Meyers, C. A. et al. Early immunomodulatory effects of implanted human perivascular stromal cells during bone formation. Tissue Eng. Part. A 24, 448–457 (2018).
Tanaka, S. et al. Sphingosine 1-phosphate signaling in perivascular cells enhances inflammation and fibrosis in the kidney. Sci. Transl. Med. 14, eabj2681 (2022).
Li, Q., Yu, Y., Bischoff, J., Mulliken, J. B. & Olsen, B. R. Differential expression of CD146 in tissues and endothelial cells derived from infantile haemangioma and normal human skin. J. Pathol. 201, 296–302 (2003).
Ozerdem, U., Grako, K. A., Dahlin-Huppe, K., Monosov, E. & Stallcup, W. B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 222, 218–227 (2001).
Nehls, V. & Drenckhahn, D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J. Cell Biol. 113, 147–154 (1991).
Lemos, D. R. et al. Interleukin-1β activates a MYC-dependent metabolic switch in kidney stromal cells necessary for progressive tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 29, 1690–1705 (2018).
Perry, H. M. et al. Perivascular CD73+ cells attenuate inflammation and interstitial fibrosis in the kidney microenvironment. Am. J. Physiol. Renal Physiol. 317, F658–F669 (2019).
Kramann, R., Wongboonsin, J., Chang-Panesso, M., Machado, F. G. & Humphreys, B. D. Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J. Am. Soc. Nephrol. 28, 776–784 (2017).
Maeda, K. et al. Identification of meflin as a potential marker for mesenchymal stromal cells. Sci. Rep. 6, 22288 (2016).
Minatoguchi, S. et al. A novel renal perivascular mesenchymal cell subset gives rise to fibroblasts distinct from classic myofibroblasts. Sci. Rep. 12, 5389 (2022).
Stefanska, A. et al. Human kidney pericytes produce renin. Kidney Int. 90, 1251–1261 (2016).
Freitas, F. & Attwell, D. Pericyte-mediated constriction of renal capillaries evokes no-reflow and kidney injury following ischaemia. eLife 11, e74211 (2022).
Crislip, G. R., O’Connor, P. M., Wei, Q. & Sullivan, J. C. Vasa recta pericyte density is negatively associated with vascular congestion in the renal medulla following ischemia reperfusion in rats. Am. J. Physiol. Renal Physiol. 313, F1097–F1105 (2017).
Kwon, O., Hong, S. M., Sutton, T. A. & Temm, C. J. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am. J. Physiol. Renal Physiol. 295, F351–F359 (2008).
Peppiatt, C. M., Howarth, C., Mobbs, P. & Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704 (2006).
Crawford, C., Wildman, S. S., Kelly, M. C., Kennedy-Lydon, T. M. & Peppiatt-Wildman, C. M. Sympathetic nerve-derived ATP regulates renal medullary vasa recta diameter via pericyte cells: a role for regulating medullary blood flow. Front. Physiol. 4, 307 (2013).
Crawford, C. et al. An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron. Physiol. 120, p17–p31 (2012).
Bruzzone, R., Hormuzdi, S. G., Barbe, M. T., Herb, A. & Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl Acad. Sci. USA 100, 13644–13649 (2003).
Jankowski, J. et al. Epithelial and endothelial pannexin1 channels mediate AKI. J. Am. Soc. Nephrol. 29, 1887–1899 (2018).
Hasegawa, S. et al. Comprehensive three-dimensional analysis (CUBIC-kidney) visualizes abnormal renal sympathetic nerves after ischemia/reperfusion injury. Kidney Int. 96, 129–138 (2019).
Alejandro, V. et al. Mechanisms of filtration failure during postischemic injury of the human kidney. A study of the reperfused renal allograft. J. Clin. Invest. 95, 820–831 (1995).
Brodsky, S. V. et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am. J. Physiol. Renal Physiol. 282, F1140–F1149 (2002).
Nangaku, M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 17, 17–25 (2006).
Tanaka, S., Tanaka, T. & Nangaku, M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Renal Physiol. 307, F1187–F1195 (2014).
Souma, T. et al. Erythropoietin synthesis in renal myofibroblasts is restored by activation of hypoxia signaling. J. Am. Soc. Nephrol. 27, 428–438 (2016).
Minamishima, Y. A. et al. Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood 111, 3236–3244 (2008).
Sato, K. et al. An immortalized cell line derived from renal erythropoietin-producing (REP) cells demonstrates their potential to transform into myofibroblasts. Sci. Rep. 9, 11254 (2019).
Besarab, A. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N. Engl. J. Med. 339, 584–590 (1998).
Drueke, T. B. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N. Engl. J. Med. 355, 2071–2084 (2006).
Pfeffer, M. A. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 361, 2019–2032 (2009).
Singh, A. K. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N. Engl. J. Med. 355, 2085–2098 (2006).
Szczech, L. A. et al. Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int. 74, 791–798 (2008).
Bernhardt, W. M. et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J. Am. Soc. Nephrol. 21, 2151–2156 (2010).
Kobayashi, H., Davidoff, O., Pujari-Palmer, S., Drevin, M. & Haase, V. H. EPO synthesis induced by HIF-PHD inhibition is dependent on myofibroblast transdifferentiation and colocalizes with non-injured nephron segments in murine kidney fibrosis. Acta Physiol. 235, e13826 (2022).
Holdstock, L. et al. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J. Am. Soc. Nephrol. 27, 1234–1244 (2016).
Sugahara, M. et al. Prolyl hydroxylase domain inhibitor protects against metabolic disorders and associated kidney disease in obese type 2 diabetic mice. J. Am. Soc. Nephrol. 31, 560–577 (2020).
Pan, S. Y. et al. Kidney pericyte hypoxia-inducible factor regulates erythropoiesis but not kidney fibrosis. Kidney Int. 99, 1354–1368 (2021).
Locatelli, F. & Del Vecchio, L. Hypoxia-inducible factor-prolyl hydroxyl domain inhibitors: from theoretical superiority to clinical noninferiority compared with current ESAs? J. Am. Soc. Nephrol. 33, 1966–1979 (2022).
Sugahara, M., Tanaka, T. & Nangaku, M. Future perspectives of anemia management in chronic kidney disease using hypoxia-inducible factor-prolyl hydroxylase inhibitors. Pharmacol. Ther. 239, 108272 (2022).
Quaggin, S. E. & Kapus, A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 80, 41–50 (2011).
Deng, Y. et al. Blocking protein phosphatase 2A signaling prevents endothelial-to-mesenchymal transition and renal fibrosis: a peptide-based drug therapy. Sci. Rep. 6, 19821 (2016).
Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M. & Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19, 2282–2287 (2008).
Phua, Y. L., Martel, N., Pennisi, D. J., Little, M. H. & Wilkinson, L. Distinct sites of renal fibrosis in Crim1 mutant mice arise from multiple cellular origins. J. Pathol. 229, 685–696 (2013).
Tang, Y., Harrington, A., Yang, X., Friesel, R. E. & Liaw, L. The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis 48, 563–567 (2010).
De Palma, M. et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226 (2005).
Cai, J., Kehoe, O., Smith, G. M., Hykin, P. & Boulton, M. E. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 49, 2163–2171 (2008).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E. & Speck, N. A. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457, 887–891 (2009).
Reich, B. et al. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 84, 78–89 (2013).
Kramann, R. et al. Parabiosis and single-cell RNA sequencing reveal a limited contribution of monocytes to myofibroblasts in kidney fibrosis. JCI Insight 3, e99561 (2018).
Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 589, 281–286 (2021).
Shaw, I. W. et al. Aging modulates the effects of ischemic injury upon mesenchymal cells within the renal interstitium and microvasculature. Stem Cell Transl. Med. 10, 1232–1248 (2021).
Zhou, D. et al. Tubule-derived wnts are required for fibroblast activation and kidney fibrosis. J. Am. Soc. Nephrol. 28, 2322–2336 (2017).
Maarouf, O. H. et al. Paracrine wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J. Am. Soc. Nephrol. 27, 781–790 (2016).
Ding, H. et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J. Am. Soc. Nephrol. 23, 801–813 (2012).
Fabian, S. L. et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am. J. Pathol. 180, 1441–1453 (2012).
Kramann, R. et al. Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J. Clin. Invest. 125, 2935–2951 (2015).
Liu, X. et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 97, 1181–1195 (2020).
Bielesz, B. et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Invest. 120, 4040–4054 (2010).
Li, H. et al. Upregulation of HER2 in tubular epithelial cell drives fibroblast activation and renal fibrosis. Kidney Int. 96, 674–688 (2019).
Dwivedi, N. et al. Epithelial vasopressin type-2 receptors regulate myofibroblasts by a yap-ccn2-dependent mechanism in polycystic kidney disease. J. Am. Soc. Nephrol. 31, 1697–1710 (2020).
Xu, X. et al. High-fidelity CRISPR/Cas9-based gene-specific hydroxymethylation rescues gene expression and attenuates renal fibrosis. Nat. Commun. 9, 3509 (2018).
Chou, Y. H. et al. Methylation in pericytes after acute injury promotes chronic kidney disease. J. Clin. Invest. 130, 4845–4857 (2020).
Tanaka, S. et al. Vascular adhesion protein-1 enhances neutrophil infiltration by generation of hydrogen peroxide in renal ischemia/reperfusion injury. Kidney Int. 92, 154–164 (2017).
Fritzemeier, R. et al. Discovery of in vivo active sphingosine-1-phosphate transporter (spns2) inhibitors. J. Med. Chem. 65, 7656–7681 (2022).
Hafizi, R. et al. Sphk1 and Sphk2 differentially regulate erythropoietin synthesis in mouse renal interstitial fibroblast-like cells. Int. J. Mol. Sci. 23, 5882 (2022).
Hafizi, R., Imeri, F., Wenger, R. H. & Huwiler, A. S1P stimulates erythropoietin production in mouse renal interstitial fibroblasts by S1P1 and S1P3 receptor activation and HIF-2α stabilization. Int. J. Mol. Sci. 22, 9467 (2021).
Pai, C. H. et al. Targeting fibroblast CD248 attenuates CCL17-expressing macrophages and tissue fibrosis. Sci. Rep. 10, 16772 (2020).
Wu, Y. et al. miR-145a regulates pericyte dysfunction in a murine model of sepsis. J. Infect. Dis. 222, 1037–1045 (2020).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Paik, Y. H. et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 37, 1043–1055 (2003).
Guo, J. et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology 49, 960–968 (2009).
Chen, Y. T. et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 80, 1170–1181 (2011).
Conway, B. R. et al. Kidney single-cell atlas reveals myeloid heterogeneity in progression and regression of kidney disease. J. Am. Soc. Nephrol. 31, 2833–2854 (2020).
Kida, Y., Ieronimakis, N., Schrimpf, C., Reyes, M. & Duffield, J. S. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J. Am. Soc. Nephrol. 24, 559–572 (2013).
Thurman, J. M. Complement in kidney disease: core curriculum 2015. Am. J. Kidney Dis. 65, 156–168 (2015).
Reis, E. S., Mastellos, D. C., Hajishengallis, G. & Lambris, J. D. New insights into the immune functions of complement. Nat. Rev. Immunol. 19, 503–516 (2019).
Kemper, C. et al. Complement: the road less traveled. J. Immunol. 210, 119–125 (2023).
Anders, H. J., Fernandez-Juarez, G. M., Vaglio, A., Romagnani, P. & Floege, J. CKD therapy to improve outcomes of immune-mediated glomerular diseases. Nephrol. Dial. Transpl. gfad069 (2023).
Merle, N. S., Church, S. E., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part I — molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 (2015).
Xavier, S. et al. Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis. Am. J. Physiol. Renal Physiol. 312, F516–F532 (2017).
Xavier, S. et al. Complement C1r serine protease contributes to kidney fibrosis. Am. J. Physiol. Renal Physiol. 317, F1293–F1304 (2019).
Portilla, D. & Xavier, S. Role of intracellular complement activation in kidney fibrosis. Br. J. Pharmacol. 178, 2880–2891 (2021).
Sahu, R. K. et al. Folic acid-mediated fibrosis is driven by C5a receptor 1-mediated activation of kidney myeloid cells. Am. J. Physiol. Renal Physiol. 322, F597–F610 (2022).
Lech, M. et al. Macrophage phenotype controls long-term AKI outcomes–kidney regeneration versus atrophy. J. Am. Soc. Nephrol. 25, 292–304 (2014).
Venkatachalam, M. A., Weinberg, J. M., Kriz, W. & Bidani, A. K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 26, 1765–1776 (2015).
Castellano, G. et al. Complement activation during ischemia/reperfusion injury induces pericyte-to-myofibroblast transdifferentiation regulating peritubular capillary lumen reduction through pERK signaling. Front. Immunol. 9, 1002 (2018).
Naba, A. et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteom. 11, M111 014647 (2012).
Wooden, B., Estebanez, B. T., Navarro-Torres, M. & Bomback, A. S. Complement inhibitors for kidney disease. Nephrol. Dial. Transpl. gfad079 https://doi.org/10.1093/ndt/gfad079 (2023).
Gerhardt, L. M. S. et al. Lineage tracing and single-nucleus multiomics reveal novel features of adaptive and maladaptive repair after acute kidney injury. J Am Soc Nephrol, 34, 554–571 (2023).
Schiessl, I. M. et al. Renal interstitial platelet-derived growth factor receptor-β cells support proximal tubular regeneration. J. Am. Soc. Nephrol. 29, 1383–1396 (2018).
Nakamura, J. et al. Myofibroblasts acquire retinoic acid-producing ability during fibroblast-to-myofibroblast transition following kidney injury. Kidney Int. 95, 526–539 (2019).
Nakagawa, T. et al. Role of PDGF B-chain and PDGF receptors in rat tubular regeneration after acute injury. Am. J. Pathol. 155, 1689–1699 (1999).
Hara, A. et al. Roles of the mesenchymal stromal/stem cell marker meflin in cardiac tissue repair and the development of diastolic dysfunction. Circ. Res. 125, 414–430 (2019).
Shi, M. et al. Effects of erythropoietin receptor activity on angiogenesis, tubular injury, and fibrosis in acute kidney injury: a “U-shaped” relationship. Am. J. Physiol. Renal Physiol. 314, F501–F516 (2018).
Zhou, D. et al. Fibroblast-specific β-catenin signaling dictates the outcome of AKI. J. Am. Soc. Nephrol. 29, 1257–1271 (2018).
Fujigaki, Y. et al. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Arch. 446, 164–176 (2005).
Sun, D. F., Fujigaki, Y., Fujimoto, T., Yonemura, K. & Hishida, A. Possible involvement of myofibroblasts in cellular recovery of uranyl acetate-induced acute renal failure in rats. Am. J. Pathol. 157, 1321–1335 (2000).
Stallcup, W. B. The NG2 proteoglycan in pericyte biology. Adv. Exp. Med. Biol. 1109, 5–19 (2018).
Jensen, A. R. et al. Neer award 2018: platelet-derived growth factor receptor α co-expression typifies a subset of platelet-derived growth factor receptor β-positive progenitor cells that contribute to fatty degeneration and fibrosis of the murine rotator cuff. J. Shoulder Elbow Surg. 27, 1149–1161 (2018).
He, L. et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 5, 180160 (2018).
Enstrom, A., Carlsson, R., Ozen, I. & Paul, G. RGS5: a novel role as a hypoxia-responsive protein that suppresses chemokinetic and chemotactic migration in brain pericytes. Biol. Open 11, bio059371 (2022).
Roth, M. et al. Regulator of G-protein signaling 5 regulates the shift from perivascular to parenchymal pericytes in the chronic phase after stroke. FASEB J. 33, 8990–8998 (2019).
Maxwell, P. H. et al. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int. 44, 1149–1162 (1993).
Rockey, D. C., Weymouth, N. & Shi, Z. Smooth muscle α actin (Acta2) and myofibroblast function during hepatic wound healing. PLoS One 8, e77166 (2013).
Acknowledgements
The authors thank D. Rosin for careful reading of the manuscript before submission. Work in the laboratory of S.T. is funded by JSPS KAKENHI (JP21K20894, JP22K16232), AMED (JP22gm6510016), the Ichiro Kanehara Foundation, MSD Life Science Foundation, the Uehara Memorial Foundation, Life Science Foundation of Japan, the Salt Science Research Foundation (2327), Research Fund of Mitsukoshi Health and Welfare Foundation 2022, and Kobayashi Foundation. Work in the laboratory of D.P. is funded by National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01 DK 12262401A1). Work in the laboratory of M.D.O. is supported by the NIDDK (R01DK085259 and R01DK123248).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to discussions of the content and wrote, reviewed or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks R. Kramann, S.-L. Lin, Y. Liu and S. Zhuang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanaka, S., Portilla, D. & Okusa, M.D. Role of perivascular cells in kidney homeostasis, inflammation, repair and fibrosis. Nat Rev Nephrol 19, 721–732 (2023). https://doi.org/10.1038/s41581-023-00752-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00752-7
This article is cited by
-
Targeting inflammation in perivascular cells and neuroimmune interactions for treating kidney disease
Clinical and Experimental Nephrology (2024)