Abstract
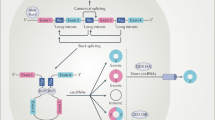
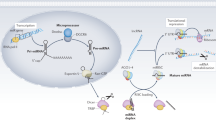
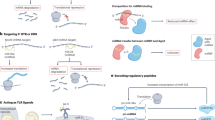
Post-transcriptional regulation by non-coding RNAs (ncRNAs) can modulate the expression of genes involved in kidney physiology and disease. A large variety of ncRNA species exist, including microRNAs, long non-coding RNAs, piwi-interacting RNAs, small nucleolar RNAs, circular RNAs and yRNAs. Despite early assumptions that some of these species may exist as by-products of cell or tissue injury, a growing body of literature suggests that these ncRNAs are functional and participate in a variety of processes. Although they function intracellularly, ncRNAs are also present in the circulation, where they are carried by extracellular vesicles, ribonucleoprotein complexes or lipoprotein complexes such as HDL. These systemic, circulating ncRNAs are derived from specific cell types and can be directly transferred to a variety of cells, including endothelial cells of the vasculature and virtually any cell type in the kidney, thereby affecting the function of the host cell and/or its response to injury. Moreover, chronic kidney disease itself, as well as injury states associated with transplantation and allograft dysfunction, is associated with a shift in the distribution of circulating ncRNAs. These findings may provide opportunities for the identification of biomarkers with which to monitor disease progression and/or the development of therapeutic interventions.
Key points
-
Circulating non-coding RNAs (ncRNAs) are mediators of cell–cell communication; evidence suggests that they have important roles in kidney disease.
-
Circulating ncRNAs are selectively loaded into their carriers; selective uptake by recipient cells suggests that transported ncRNAs may have very specific functions in specific cell types.
-
ncRNAs in specific carriers may serve as better biomarkers than total levels of circulating ncRNAs.
-
A variety of circulating ncRNA species beyond microRNAs, including long non-coding RNAs, piwi-interacting RNAs, small nucleolar RNAs, circular RNAs and yRNAs, as well as tRNA fragments, may have important functions, but their role in kidney physiology and disease remains to be explored.
-
Selective modulation of carrier–ncRNA complexes may provide novel therapeutic targeting strategies for various kidney diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Encode Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
The ENCODE Project Consortium. The ENCODE (ENCyclopedia Of DNA Elements) Project. Science 306, 636–640 (2004).
Mattick, J. S. The central role of RNA in human development and cognition. FEBS Lett. 585, 1600–1616 (2011).
Hube, F. & Francastel, C. Mammalian introns: when the junk generates molecular diversity. Int. J. Mol. Sci. 16, 4429–4452 (2015).
Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 12, 861–874 (2011).
Gebeshuber, C. A. et al. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat. Med. 19, 481–487 (2013).
Kato, M. et al. An endoplasmic reticulum stress-regulated lncRNA hosting a microRNA megacluster induces early features of diabetic nephropathy. Nat. Commun. 7, 12864 (2016).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Arroyo, J. D. et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl Acad. Sci. USA 108, 5003–5008 (2011).
Vickers, K. C., Palmisano, B. T., Shoucri, B. M., Shamburek, R. D. & Remaley, A. T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 13, 423–433 (2011).
Creemers, E. E., Tijsen, A. J. & Pinto, Y. M. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 110, 483–495 (2012).
Yeri, A. et al. Total extracellular small RNA profiles from plasma, saliva, and urine of healthy subjects. Sci. Rep. 7, 44061 (2017).
Srinivasan, S. et al. Small RNA sequencing across diverse biofluids identifies optimal methods for exRNA isolation. Cell 177, 446–462 e416 (2019).
Shi, J. et al. PANDORA-seq expands the repertoire of regulatory small RNAs by overcoming RNA modifications. Nat. Cell Biol. 23, 424–436 (2021).
Ambros, V. The functions of animal microRNAs. Nature 431, 350–355 (2004).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
van Zonneveld, A. J., Rabelink, T. J. & Bijkerk, R. miRNA-coordinated networks as promising therapeutic targets for acute kidney injury. Am. J. Pathol. 187, 20–24 (2017).
Stefani, G. & Slack, F. J. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 9, 219–230 (2008).
Mahtal, N., Lenoir, O., Tinel, C., Anglicheau, D. & Tharaux, P. L. MicroRNAs in kidney injury and disease. Nat. Rev. Nephrol. 18, 643–662 (2022).
Amaral, P. P. & Mattick, J. S. Noncoding RNA in development. Mamm. Genome 19, 454–492 (2008).
Yao, R. W., Wang, Y. & Chen, L. L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 21, 542–551 (2019).
Jia, H. et al. Genome-wide computational identification and manual annotation of human long noncoding RNA genes. RNA 16, 1478–1487 (2010).
Moreno, J. A. et al. Non-coding RNAs in kidney diseases: the long and short of them. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22116077 (2021).
Lorenzen, J. M. & Thum, T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat. Rev. Nephrol. 12, 360–373 (2016).
Memczak, S. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Patop, I. L., Wust, S. & Kadener, S. Past, present, and future of circRNAs. EMBO J. 38, e100836 (2019).
van Zonneveld, A. J., Kolling, M., Bijkerk, R. & Lorenzen, J. M. Circular RNAs in kidney disease and cancer. Nat. Rev. Nephrol. 17, 814–826 (2021).
Pruijn, G. J., Wingens, P. A., Peters, S. L., Thijssen, J. P. & van Venrooij, W. J. Ro RNP associated Y RNAs are highly conserved among mammals. Biochim. Biophys. Acta 1216, 395–401 (1993).
Boccitto, M. & Wolin, S. L. Ro60 and Y RNAs: structure, functions, and roles in autoimmunity. Crit. Rev. Biochem. Mol. Biol. 54, 133–152 (2019).
Kowalski, M. P. & Krude, T. Functional roles of non-coding Y RNAs. Int. J. Biochem. Cell Biol. 66, 20–29 (2015).
Driedonks, T. A. P. & Nolte-‘t Hoen, E. N. M. Circulating Y-RNAs in extracellular vesicles and ribonucleoprotein complexes; implications for the immune system. Front. Immunol. 9, 3164 (2018).
Thomson, D. W. et al. Assessing the gene regulatory properties of argonaute-bound small RNAs of diverse genomic origin. Nucleic Acids Res. 43, 470–481 (2015).
Shen, Y. et al. Transfer RNA-derived fragments and tRNA halves: biogenesis, biological functions and their roles in diseases. J. Mol. Med. 96, 1167–1176 (2018).
Honda, S. et al. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc. Natl Acad. Sci. USA 112, E3816–E3825 (2015).
Chen, Q. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).
Tosar, J. P. & Cayota, A. Extracellular tRNAs and tRNA-derived fragments. RNA Biol. 17, 1149–1167 (2020).
Nechooshtan, G., Yunusov, D., Chang, K. & Gingeras, T. R. Processing by RNase 1 forms tRNA halves and distinct Y RNA fragments in the extracellular environment. Nucleic Acids Res. 48, 8035–8049 (2020).
Li, D. et al. Role of tRNA derived fragments in renal ischemia-reperfusion injury. Ren. Fail. 44, 815–825 (2022).
Pan, X. et al. Transfer RNA fragments in the kidney in hypertension. Hypertension 77, 1627–1637 (2021).
Williams, A. M. et al. Histologically resolved small RNA maps in primary focal segmental glomerulosclerosis indicate progressive changes within glomerular and tubulointerstitial regions. Kidney Int. 101, 766–778 (2022).
Ozata, D. M., Gainetdinov, I., Zoch, A., O’Carroll, D. & Zamore, P. D. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. 20, 89–108 (2019).
Kiss, T. Small nucleolar RNA-guided post-transcriptional modification of cellular RNAs. EMBO J. 20, 3617–3622 (2001).
Ender, C. et al. A human snoRNA with microRNA-like functions. Mol. Cell 32, 519–528 (2008).
Freedman, J. E. et al. Diverse human extracellular RNAs are widely detected in human plasma. Nat. Commun. 7, 11106 (2016).
Persson, H. et al. The non-coding RNA of the multidrug resistance-linked vault particle encodes multiple regulatory small RNAs. Nat. Cell Biol. 11, 1268–1271 (2009).
Fica, S. M. et al. RNA catalyses nuclear pre-mRNA splicing. Nature 503, 229–234 (2013).
Allen, R. M. et al. Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins. J. Extracell. Vesicles 7, 1506198 (2018).
Wang, K. et al. The complex exogenous RNA spectra in human plasma: an interface with human gut biota. PLoS One 7, e51009 (2012).
Dellar, E. R., Hill, C., Melling, G. E., Carter, D. R. F. & Baena-Lopez, L. A. Unpacking extracellular vesicles: RNA cargo loading and function. J. Extracell. Biol. 1, e40 (2022).
Borges, F. T. et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 24, 385–392 (2013).
Abbasian, N., Herbert, K. E., Pawluczyk, I., Burton, J. O. & Bevington, A. Vesicles bearing gifts: the functional importance of micro-RNA transfer in extracellular vesicles in chronic kidney disease. Am. J. Physiol. Renal Physiol. 315, F1430–F1443 (2018).
Thomou, T. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455 (2017).
Li, S. et al. Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol. Biochem. 39, 2439–2450 (2016).
Nolte-‘t Hoen, E. N. et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 40, 9272–9285 (2012).
van Balkom, B. W., Eisele, A. S., Pegtel, D. M., Bervoets, S. & Verhaar, M. C. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J. Extracell. Vesicles 4, 26760 (2015).
Preusser, C. et al. Selective release of circRNAs in platelet-derived extracellular vesicles. J. Extracell. Vesicles 7, 1424473 (2018).
Tosar, J. P. et al. Assessment of small RNA sorting into different extracellular fractions revealed by high-throughput sequencing of breast cell lines. Nucleic Acids Res. 43, 5601–5616 (2015).
Garcia-Martin, R. et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 601, 446–451 (2022).
Mulcahy, L. A., Pink, R. C. & Carter, D. R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles https://doi.org/10.3402/jev.v3.24641 (2014).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Vinas, J. L. et al. Receptor-ligand interaction mediates targeting of endothelial colony forming cell-derived exosomes to the kidney after ischemic injury. Sci. Rep. 8, 16320 (2018).
Kholia, S. et al. Human liver stem cell derived extracellular vesicles alleviate kidney fibrosis by interfering with the β-catenin pathway through miR29b. Int. J. Mol. Sci. https://doi.org/10.3390/ijms221910780 (2021).
Gao, M. et al. Liver-derived exosome-laden lncRNA MT1DP aggravates cadmium-induced nephrotoxicity. Environ. Pollut. 258, 113717 (2020).
Matthews, O. et al. Transfer of hepatocellular microRNA regulates cytochrome P450 2E1 in renal tubular cells. EBioMedicine 62, 103092 (2020).
Gao, L. et al. Cardio-renal exosomes in myocardial infarction serum regulate proangiogenic paracrine signaling in adipose mesenchymal stem cells. Theranostics 10, 1060–1073 (2020).
Zhang, Q. et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell Biol. 23, 1240–1254 (2021).
Zhang, H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332–343 (2018).
Zhang, Q. et al. Transfer of functional cargo in exomeres. Cell Rep. 27, 940–954.e946 (2019).
Yuana, Y., Levels, J., Grootemaat, A., Sturk, A. & Nieuwland, R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J. Extracell. Vesicles https://doi.org/10.3402/jev.v3.23262 (2014).
Vickers, K. C. & Michell, D. L. HDL-small RNA export, transport, and functional delivery in atherosclerosis. Curr. Atheroscler. Rep. 23, 38 (2021).
Wagner, J. et al. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler. Thromb. Vasc. Biol. 33, 1392–1400 (2013).
Baragetti, A. et al. High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J. Intern. Med. 274, 252–262 (2013).
Nam, K. H. et al. Association between serum high-density lipoprotein cholesterol levels and progression of chronic kidney disease: results from the KNOW-CKD. J. Am. Heart Assoc. 8, e011162 (2019).
Davis, C. E. et al. Sex difference in high density lipoprotein cholesterol in six countries. Am. J. Epidemiol. 143, 1100–1106 (1996).
Tosar, J. P., Witwer, K. & Cayota, A. Revisiting extracellular RNA release, processing, and function. Trends Biochem. Sci. 46, 438–445 (2021).
McKenzie, A. J. et al. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 15, 978–987 (2016).
Turchinovich, A. & Burwinkel, B. Distinct AGO1 and AGO2 associated miRNA profiles in human cells and blood plasma. RNA Biol. 9, 1066–1075 (2012).
Cantaluppi, V. et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 82, 412–427 (2012).
Bitzer, M., Ben-Dov, I. Z. & Thum, T. Microparticles and microRNAs of endothelial progenitor cells ameliorate acute kidney injury. Kidney Int. 82, 375–377 (2012).
Bijkerk, R. et al. Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J. Am. Soc. Nephrol. 25, 1710–1722 (2014).
He, Z., Wang, H. & Yue, L. Endothelial progenitor cells-secreted extracellular vesicles containing microRNA-93-5p confer protection against sepsis-induced acute kidney injury via the KDM6B/H3K27me3/TNF-alpha axis. Exp. Cell Res. 395, 112173 (2020).
Zhou, Y. et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol. Ther. 26, 1375–1384 (2018).
Pan, T. et al. Delayed remote ischemic preconditioning confers renoprotection against septic acute kidney injury via exosomal miR-21. Theranostics 9, 405–423 (2019).
Vinas, J. L. et al. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int. 90, 1238–1250 (2016).
Cavallari, C. et al. Online hemodiafiltration inhibits inflammation-related endothelial dysfunction and vascular calcification of uremic patients modulating miR-223 expression in plasma extracellular vesicles. J. Immunol. 202, 2372–2383 (2019).
Uil, M. et al. Cellular origin and microRNA profiles of circulating extracellular vesicles in different stages of diabetic nephropathy. Clin. Kidney J. 14, 358–365 (2021).
Zietzer, A. et al. MicroRNA-mediated vascular intercellular communication is altered in chronic kidney disease. Cardiovasc. Res. 118, 316–333 (2022).
Hanatani, S. et al. Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J. Am. Soc. Nephrol. 25, 2800–2811 (2014).
Wang, B. et al. miR-26a limits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics 9, 1864–1877 (2019).
Zhang, A. et al. Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease. FASEB J. 33, 13590–13601 (2019).
Wang, H. et al. Exosome-mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol. Ther. 27, 571–583 (2019).
Guan, H. et al. Injured tubular epithelial cells activate fibroblasts to promote kidney fibrosis through miR-150-containing exosomes. Exp. Cell Res. 392, 112007 (2020).
Zhao, S. et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics 11, 8660–8673 (2021).
Zhou, X. et al. Tubular cell-derived exosomal miR-150-5p contributes to renal fibrosis following unilateral ischemia-reperfusion injury by activating fibroblast in vitro and in vivo. Int. J. Biol. Sci. 17, 4021–4033 (2021).
Lu, Q. et al. Circulating miR-103a-3p contributes to angiotensin II-induced renal inflammation and fibrosis via a SNRK/NF-κB/p65 regulatory axis. Nat. Commun. 10, 2145 (2019).
Su, H. et al. Podocyte-derived extracellular vesicles mediate renal proximal tubule cells dedifferentiation via microRNA-221 in diabetic nephropathy. Mol. Cell Endocrinol. 518, 111034 (2020).
Hill, N. et al. Glomerular endothelial derived vesicles mediate podocyte dysfunction: A potential role for miRNA. PLoS One 15, e0224852 (2020).
Huang, H. et al. M2 macrophage-derived exosomal miR-25-3p improves high glucose-induced podocytes injury through activation autophagy via inhibiting DUSP1 expression. IUBMB Life 72, 2651–2662 (2020).
Zhuang, Y., Zheng, H., Yang, Y. & Ni, H. GABA alleviates high glucose-induced podocyte injury through dynamically altering the expression of macrophage M1/M2-derived exosomal miR-21a-5p/miR-25-3p. Biochem. Biophys. Res. Commun. 618, 38–45 (2022).
Ding, X. et al. MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J. Endocrinol. Invest. 44, 1175–1184 (2021).
Wang, Z., Sun, W., Li, R. & Liu, Y. miRNA-93-5p in exosomes derived from M2 macrophages improves lipopolysaccharide-induced podocyte apoptosis by targeting Toll-like receptor 4. Bioengineered 13, 7683–7696 (2022).
Zhang, Z. et al. Macrophage-derived exosomal miRNA-155 promotes tubular injury in ischemia-induced acute kidney injury. Int. J. Mol. Med. https://doi.org/10.3892/ijmm.2022.5172 (2022).
Zhao, J., Chen, J., Zhu, W., Qi, X. M. & Wu, Y. G. Exosomal miR-7002-5p derived from high glucose-induced macrophages suppresses autophagy in tubular epithelial cells by targeting Atg9b. FASEB J. 36, e22501 (2022).
Li, Z. L. et al. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 95, 388–404 (2019).
Lv, L. L. et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 27, 210–226 (2020).
Ding, H., Li, L. X., Harris, P. C., Yang, J. & Li, X. Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease. Nat. Commun. 12, 4548 (2021).
Franzin, R. et al. Extracellular vesicles derived from patients with antibody-mediated rejection induce tubular senescence and endothelial to mesenchymal transition in renal cells. Am. J. Transpl. 22, 2139–2157 (2022).
Rutman, A. K. et al. Extracellular vesicles from kidney allografts express miR-218-5p and Alter Th17/Treg ratios. Front. Immunol. 13, 784374 (2022).
Pang, X. L. et al. Immature dendritic cells derived exosomes promotes immune tolerance by regulating T cell differentiation in renal transplantation. Aging 11, 8911–8924 (2019).
Wang, B. et al. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. 24, 1290–1301 (2016).
Yang, Y. et al. Exosomes derived from mesenchymal stem cells ameliorate renal fibrosis via delivery of miR-186-5p. Hum. Cell 35, 83–97 (2022).
Xu, S. et al. Bone marrow mesenchymal stem cell-derived exosomal miR-21a-5p alleviates renal fibrosis by attenuating glycolysis by targeting PFKM. Cell Death Dis. 13, 876 (2022).
Wang, S. J. et al. Bone marrow mesenchymal stem cell-derived extracellular vesicles containing miR-181d protect rats against renal fibrosis by inhibiting KLF6 and the NF-κB signaling pathway. Cell Death Dis. 13, 535 (2022).
Hu, X. et al. Bone marrow mesenchymal stem cell-derived exosomal miR-34c-5p ameliorates RIF by inhibiting the core fucosylation of multiple proteins. Mol. Ther. 30, 763–781 (2022).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Cao, J. Y. et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 11, 5248–5266 (2021).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010). 531p following 143.
Zhu, G. et al. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J. Cell Physiol. 234, 23736–23749 (2019).
Li, X. et al. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics 10, 9561–9578 (2020).
Wu, X. et al. Exosomes secreted by mesenchymal stem cells induce immune tolerance to mouse kidney transplantation via transporting LncRNA DANCR. Inflammation 45, 460–475 (2022).
Wang, Z. G. et al. Donor BMSC-derived small extracellular vesicles relieve acute rejection post-renal allograft through transmitting Loc108349490 to dendritic cells. Aging Cell 20, e13461 (2021).
Cao, S. et al. Circular RNA mmu_circ_0001295 from hypoxia pretreated adipose-derived mesenchymal stem cells (ADSCs) exosomes improves outcomes and inhibits sepsis-induced renal injury in a mouse model of sepsis. Bioengineered 13, 6323–6331 (2022).
Bai, S. et al. Exosomal circ_DLGAP4 promotes diabetic kidney disease progression by sponging miR-143 and targeting ERBB3/NF-κB/MMP-2 axis. Cell Death Dis. 11, 1008 (2020).
Canfran-Duque, A., Lin, C. S., Goedeke, L., Suarez, Y. & Fernandez-Hernando, C. Micro-RNAs and high-density lipoprotein metabolism. Arterioscler. Thromb. Vasc. Biol. 36, 1076–1084 (2016).
Hennessy, E. J. et al. The long noncoding RNA CHROME regulates cholesterol homeostasis in primate. Nat. Metab. 1, 98–110 (2019).
Tabet, F. et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 5, 3292 (2014).
Bijkerk, R. et al. Circulating microRNAs associate with diabetic nephropathy and systemic microvascular damage and normalize after simultaneous pancreas-kidney transplantation. Am. J. Transpl. 15, 1081–1090 (2015).
Hourigan, S. T. et al. The regulation of miRNAs by reconstituted high-density lipoproteins in diabetes-impaired angiogenesis. Sci. Rep. 8, 13596 (2018).
Morrison, K. R. et al. Elevated HDL-bound miR-181c-5p level is associated with diabetic vascular complications in Australian Aboriginal people. Diabetologia 64, 1402–1411 (2021).
Florijn, B. W. et al. Diabetic nephropathy alters the distribution of circulating angiogenic microRNAs among extracellular vesicles, HDL, and Ago-2. Diabetes 68, 2287–2300 (2019).
Shroff, R. et al. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J. Am. Soc. Nephrol. 25, 2658–2668 (2014).
Keene, D., Price, C., Shun-Shin, M. J. & Francis, D. P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ 349, g4379 (2014).
Laffont, B. et al. Activated platelets can deliver mRNA regulatory Ago2*microRNA complexes to endothelial cells via microparticles. Blood 122, 253–261 (2013).
Groeneweg, K. E. et al. Diabetic nephropathy alters circulating long noncoding RNA levels that normalize following simultaneous pancreas-kidney transplantation. Am. J. Transpl. 20, 3451–3461 (2020).
Gu, Y. Y. et al. Non-coding RNAs as biomarkers and therapeutic targets for diabetic kidney disease. Front. Pharmacol. 11, 583528 (2020).
Satake, E. et al. Comprehensive search for novel circulating miRNAs and Axon guidance pathway proteins associated with risk of ESKD in diabetes. J. Am. Soc. Nephrol. 32, 2331–2351 (2021).
Pezzolesi, M. G. et al. Circulating TGF-β1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes 64, 3285–3293 (2015).
Kolling, M. et al. The circular RNA ciRs-126 predicts survival in critically ill patients with acute kidney injury. Kidney Int. Rep. 3, 1144–1152 (2018).
Phulkerd, T. et al. Circulating and urinary microRNAs profile for predicting renal recovery from severe acute kidney injury. J. Intensive Care 10, 45 (2022).
Chen, Y., Han, X., Sun, Y., He, X. & Xue, D. A circulating exosomal microRNA panel as a novel biomarker for monitoring post-transplant renal graft function. J. Cell Mol. Med. 24, 12154–12163 (2020).
Kuscu, C. et al. Integrative analyses of circulating small RNAs and kidney graft transcriptome in transplant glomerulopathy. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22126218 (2021).
Mishima, E. et al. Conformational change in transfer RNA is an early indicator of acute cellular damage. J. Am. Soc. Nephrol. 25, 2316–2326 (2014).
Holderied, A. & Anders, H. J. t-RNA fragmentation as an early biomarker of (kidney) injury. J. Am. Soc. Nephrol. 25, 2145–2147 (2014).
Vallabhajosyula, P. et al. Tissue-specific exosome biomarkers for noninvasively monitoring immunologic rejection of transplanted tissue. J. Clin. Invest. 127, 1375–1391 (2017).
Gremmels, H. et al. The small RNA repertoire of small extracellular vesicles isolated from donor kidney preservation fluid provides a source for biomarker discovery for organ quality and posttransplantation graft function. Transpl. Direct 5, e484 (2019).
Driedonks, T. A. et al. Y-RNA subtype ratios in plasma extracellular vesicles are cell type-specific and are candidate biomarkers for inflammatory diseases. J. Extracell. Vesicles 9, 1764213 (2020).
Saejong, S. et al. MicroRNA-21 in plasma exosome, but not from whole plasma, as a biomarker for the severe interstitial fibrosis and tubular atrophy (IF/TA) in post-renal transplantation. Asian Pac. J. Allergy Immunol. 40, 94–102 (2022).
Hart, L. et al. Circulating small non-coding RNAs associate with kidney function in people with type 2 diabetes. Preprint at medRxiv https://doi.org/10.1101/2022.05.23.22275440 (2022).
Jankowski, J., Floege, J., Fliser, D., Bohm, M. & Marx, N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 143, 1157–1172 (2021).
Niculescu, L. S. et al. MiR-486 and miR-92a identified in circulating HDL discriminate between stable and vulnerable coronary artery disease patients. PLoS One 10, e0140958 (2015).
Meiri, E. et al. Differential expression of microRNA in serum fractions and association of Argonaute 1 microRNAs with heart failure. J. Cell Mol. Med. 24, 6586–6595 (2020).
Perez-Hernandez, J. et al. Urinary- and plasma-derived exosomes reveal a distinct microRNA signature associated with albuminuria in hypertension. Hypertension 77, 960–971 (2021).
Riffo-Campos, A. L. et al. Exosomal and plasma non-coding RNA signature associated with urinary albumin excretion in hypertension. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23020823 (2022).
Statello, L., Guo, C. J., Chen, L. L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118 (2021).
Garrelfs, S. F. et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N. Engl. J. Med. 384, 1216–1226 (2021).
Regulus Therapeutics Inc. A study of RGLS8429 in patients with autosomal dominant polycystic kidney disease. https://clinicaltrials.gov/ct2/show/NCT05521191 (2023).
Genzyme. Study of lademirsen (SAR339375) in patients with Alport syndrome (HERA). https://clinicaltrials.gov/ct2/show/NCT02855268 (2022).
Herrmann, I. K., Wood, M. J. A. & Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759 (2021).
Shah, A. M. & Giacca, M. Small non-coding RNA therapeutics for cardiovascular disease. Eur. Heart J. 43, 4548–4561 (2022).
Bondue, T., van den Heuvel, L., Levtchenko, E. & Brock, R. The potential of RNA-based therapy for kidney diseases. Pediatr. Nephrol. 38, 327–344 (2022).
Tang, T. T. et al. Kim-1 targeted extracellular vesicles: a new therapeutic platform for RNAi to treat AKI. J. Am. Soc. Nephrol. 32, 2467–2483 (2021).
Zheng, W. et al. Cell-specific targeting of extracellular vesicles though engineering the glycocalyx. J. Extracell. Vesicles 11, e12290 (2022).
Nogrady, B. The challenge of delivering RNA-interference therapeutics to their target cells. Nature 574, S8–S9 (2019).
Midgley, A. C. et al. Multifunctional natural polymer nanoparticles as antifibrotic gene carriers for CKD therapy. J. Am. Soc. Nephrol. 31, 2292–2311 (2020).
Huang, S. et al. Application of ultrasound-targeted microbubble destruction-mediated exogenous gene transfer in treating various renal diseases. Hum. Gene Ther. 30, 127–138 (2019).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Winek, K. et al. Transfer RNA fragments replace microRNA regulators of the cholinergic poststroke immune blockade. Proc. Natl Acad. Sci. USA 117, 32606–32616 (2020).
Acknowledgements
The authors acknowledge funding from the Dutch Kidney Foundation (KOLLF grant 20OK015, to R.B.) and the European Foundation for the Study of Diabetes (to A.J.V.Z. and R.B.). The authors also thank Manon Zuurmond (LUMC, Leiden, the Netherlands) for generating the concept figures used in this Review.
Author information
Authors and Affiliations
Contributions
Q.Z. and R.B researched data for the article. All authors contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Benjamin Humphreys, Valérie Metzinger-Le Meuth and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Zonneveld, A.J., Zhao, Q., Rotmans, J.I. et al. Circulating non-coding RNAs in chronic kidney disease and its complications. Nat Rev Nephrol 19, 573–586 (2023). https://doi.org/10.1038/s41581-023-00725-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00725-w
This article is cited by
-
The complexity of nicotinamide adenine dinucleotide (NAD), hypoxic, and aryl hydrocarbon receptor cell signaling in chronic kidney disease
Journal of Translational Medicine (2023)