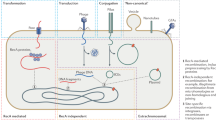
Abstract
Horizontal gene transfer (HGT), or lateral gene transfer, is the non-sexual movement of genetic information between genomes. It has played a pronounced part in bacterial and archaeal evolution, but its role in eukaryotes is less clear. Behaviours unique to eukaryotic cells — phagocytosis and endosymbiosis — have been proposed to increase the frequency of HGT, but nuclear genomes encode fewer HGTs than bacteria and archaea. Here, I review the existing theory in the context of the growing body of data on HGT in eukaryotes, which suggests that any increased chance of acquiring new genes through phagocytosis and endosymbiosis is offset by a reduced need for these genes in eukaryotes, because selection in most eukaryotes operates on variation not readily generated by HGT.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Smith, M. W., Feng, D. F. & Doolittle, R. F. Evolution by acquisition: the case for horizontal gene transfers. Trends Biochem. Sci. 17, 489–493 (1992).
Doolittle, W. F. Phylogenetic classification and the universal tree. Science 284, 2124–2129 (1999).
Ochman, H., Lawrence, J. G. & Groisman, E. A. Lateral gene transfer and the nature of bacterial innovation. Nature 405, 299–304 (2000).
Koonin, E. V., Makarova, K. S. & Aravind, L. Horizontal gene transfer in prokaryotes: quantification and classification. Annu. Rev. Microbiol. 55, 709–742 (2001).
Blais, C. & Archibald, J. M. The past, present and future of the tree of life. Curr. Biol. 31, R314–R321 (2021).
Soucy, S. M., Huang, J. & Gogarten, J. P. Horizontal gene transfer: building the web of life. Nat. Rev. Genet. 16, 472–482 (2015).
Daubin, V. & Szöllősi, G. J. Horizontal gene transfer and the history of life. Cold Spring Harb. Perspect. Biol. 8, a018036 (2016).
Domingues, S. & Nielsen, K. M. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr. Opin. Microbiol. 38, 16–21 (2017).
Brito, I. L. Examining horizontal gene transfer in microbial communities. Nat. Rev. Microbiol. 19, 442–453 (2021).
Arnold, B. J., Huang, I. T. & Hanage, W. P. Horizontal gene transfer and adaptive evolution in bacteria. Nat. Rev. Microbiol. 20, 206–218 (2022).
Gophna, U. & Altman-Price, N. Horizontal gene transfer in archaea — from mechanisms to genome evolution. Annu. Rev. Microbiol. 76, 481–502 (2022).
Ku, C. et al. Endosymbiotic gene transfer from prokaryotic pangenomes: inherited chimerism in eukaryotes. Proc. Natl Acad. Sci. USA 112, 10139–10146 (2015).
Martin, W. F. Too much eukaryote LGT. Bioessays https://doi.org/10.1002/bies.201700115 (2017). This paper and Leger et al. are endpoints of a long debate about whether HGT took place in nuclear genomes, with this article detailing arguments against the presence of widespread HGT.
Leger, M. M., Eme, L., Stairs, C. W. & Roger, A. J. Demystifying eukaryote lateral gene transfer (Response to Martin 2017 DOI: 10.1002/bies.201700115). Bioessays 40, e1700242 (2018). This paper and Martin (2017) are endpoints of a long debate about whether HGT took place in nuclear genomes, with this article detailing arguments for the presence of widespread HGT.
Martin, W. F. Eukaryote lateral gene transfer is Lamarckian. Nat. Ecol. Evol. 2, 754 (2018).
Van Etten, J. & Bhattacharya, D. Horizontal gene transfer in eukaryotes: not if, but how much? Trends Genet. 36, 915–925 (2020). This Review aims to turn the page on the debate from whether HGT affected eukaryotes to focusing on how it changed their ecology and evolution.
Fleischmann, R. D. et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269, 496–512 (1995).
Goffeau, A. et al. Life with 6000 genes. Science 274, 546–567 (1996).
del Campo, J. et al. The others: our biased perspective of eukaryotic genomes. Trends Ecol. Evol. 29, 252–259 (2014).
Keeling, P. J. & Campo, J. D. Marine protists are not just big bacteria. Curr. Biol. 27, R541–R549 (2017).
Gray, M. W. & Doolittle, W. F. Has the endosymbiont hypothesis been proven? Microbiol. Rev. 46, 1–42 (1982).
Bergthorsson, U., Richardson, A. O., Young, G. J., Goertzen, L. R. & Palmer, J. D. Massive horizontal transfer of mitochondrial genes from diverse land plant donors to the basal angiosperm Amborella. Proc. Natl Acad. Sci. USA 101, 17747–17752 (2004).
Rice, D. W. & Palmer, J. D. An exceptional horizontal gene transfer in plastids: gene replacement by a distant bacterial paralog and evidence that haptophyte and cryptophyte plastids are sisters. BMC Biol. 4, 31 (2006).
Khan, H. et al. Plastid genome sequence of the cryptophyte alga Rhodomonas salina CCMP1319: lateral transfer of putative DNA replication machinery and a test of chromist plastid phylogeny. Mol. Biol. Evol. 24, 1832–1842 (2007).
Keeling, P. J. & Palmer, J. D. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 9, 605–618 (2008).
Zhao, N., Wang, Y. & Hua, J. The roles of mitochondrion in intergenomic gene transfer in plants: a source and a pool. Int. J. Mol. Sci. 19, 547 (2018).
Filip, E. & Skuza, L. Horizontal gene transfer involving chloroplasts. Int. J. Mol. Sci. 22, 4484 (2021).
Jenkins, C. et al. Genes for the cytoskeletal protein tubulin in the bacterial genus Prosthecobacter. Proc. Natl Acad. Sci. USA 99, 17049–17054 (2002).
Guljamow, A. et al. Horizontal gene transfer of two cytoskeletal elements from a eukaryote to a cyanobacterium. Curr. Biol. 17, R757–R759 (2007).
Zaremba-Niedzwiedzka, K. et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 541, 353–358 (2017).
Imachi, H. et al. Isolation of an archaeon at the prokaryote–eukaryote interface. Nature 577, 519–525 (2020).
Akil, C. et al. Structural and biochemical evidence for the emergence of a calcium-regulated actin cytoskeleton prior to eukaryogenesis. Commun. Biol. 5, 890 (2022).
Shiratori, T., Suzuki, S., Kakizawa, Y. & Ishida, K. I. Phagocytosis-like cell engulfment by a planctomycete bacterium. Nat. Commun. 10, 5529 (2019).
Spang, A. et al. Asgard archaea are the closest prokaryotic relatives of eukaryotes. PLoS Genet. 14, e1007080 (2018).
Worden, A. Z. et al. Rethinking the marine carbon cycle: factoring in the multifarious lifestyles of microbes. Science 347, 1257594 (2015).
Husnik, F. et al. Bacterial and archaeal symbioses with protists. Curr. Biol. 31, R862–R877 (2021). This paper provides a detailed summary of the extent of symbiosis across the tree of eukaryotes, emphasizing not only how widespread it is but also how it affects genome evolution and how little we understand it functionally.
Husnik, F. et al. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 153, 1567–1578 (2013).
Husnik, F. & McCutcheon, J. P. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc. Natl Acad. Sci. USA 113, E5416–E5424 (2016).
Timmis, J. N., Ayliffe, M. A., Huang, C. Y. & Martin, W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 5, 123–135 (2004).
Stanier, R. Y. in Organization and Control in Prokaryotic and Eukaryotic Cells: 20th symposium of the Society for General Microbiology (eds Charles, H. P. & Knight, B. D.) 1–38 (Cambridge Univ. Press, 1970).
Martin, W. & Müller, M. The hydrogen hypothesis for the first eukaryote. Nature 392, 37–41 (1998).
Keeling, P. J. The impact of history on our perception of evolutionary events: endosymbiosis and the origin of eukaryotic complexity. Cold Spring Harb. Perspect. Biol. 6, a016196 (2014).
Koonin, E. V. Origin of eukaryotes from within archaea, archaeal eukaryome and bursts of gene gain: eukaryogenesis just made easier? Philos. Trans. R. Soc. B 370, 20140333 (2015).
Martin, W. F., Garg, S. & Zimorski, V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. B 370, 20140330 (2015).
Pittis, A. A. & Gabaldon, T. Late acquisition of mitochondria by a host with chimaeric prokaryotic ancestry. Nature 531, 101–104 (2016).
Martin, W. F. et al. Late mitochondrial origin is an artifact. Genome Biol. Evol. 9, 373–379 (2017).
Martin, W. F., Tielens, A. G. M., Mentel, M., Garg, S. G. & Gould, S. B. The Physiology of phagocytosis in the context of mitochondrial origin. Microbiol. Mol. Biol. Rev. 81, e00008-17 (2017).
Gabaldón, T. Relative timing of mitochondrial endosymbiosis and the “pre-mitochondrial symbioses” hypothesis. IUBMB Life 70, 1188–1196 (2018).
Gabaldon, T. Origin and early evolution of the eukaryotic cell. Annu. Rev. Microbiol. 75, 631–647 (2021).
Albers, S., Ashmore, J., Pollard, T., Spang, A. & Zhou, J. Origin of eukaryotes: what can be learned from the first successfully isolated Asgard archaeon. Fac. Rev. 11, 3 (2022).
Lander, E. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Boothby, T. C. et al. Evidence for extensive horizontal gene transfer from the draft genome of a tardigrade. Proc. Natl Acad. Sci. USA 112, 15976–15981 (2015).
Choi, I. G. & Kim, S. H. Global extent of horizontal gene transfer. Proc. Natl Acad. Sci. USA 104, 4489–4494 (2007).
Alsmark, C. et al. Patterns of prokaryotic lateral gene transfers affecting parasitic microbial eukaryotes. Genome Biol. 14, R19 (2013).
Boto, L. Horizontal gene transfer in the acquisition of novel traits by metazoans. Proc. Biol. Sci. 281, 20132450 (2014).
Schönknecht, G., Weber, A. P. & Lercher, M. J. Horizontal gene acquisitions by eukaryotes as drivers of adaptive evolution. Bioessays 36, 9–20 (2014).
Gluck-Thaler, E. & Slot, J. C. Dimensions of horizontal gene transfer in eukaryotic microbial pathogens. PLoS Pathog. 11, e1005156 (2015).
Lacroix, B. & Citovsky, V. A functional bacterium-to-plant DNA transfer machinery of Rhizobium etli. PLoS Pathog. 12, e1005502 (2016).
Husnik, F. & McCutcheon, J. P. Functional horizontal gene transfer from bacteria to eukaryotes. Nat. Rev. Microbiol. 16, 67–79 (2018). This paper provides a thorough review of bacterial-to-eukaryotic HGT, placing a much needed emphasis on functional transfers that will persist in evolutionary time, as distinguished from the simple non-functional uptake of DNA.
Fan, X. et al. Phytoplankton pangenome reveals extensive prokaryotic horizontal gene transfer of diverse functions. Sci. Adv. 6, eaba0111 (2020). This paper describes a genomic analysis including a wide diversity of ecologically important algae, showing that their genomes have been impacted to varying degrees by HGT, but that in all cases physiologically important functions have been affected.
Gabaldón, T. Patterns and impacts of nonvertical evolution in eukaryotes: a paradigm shift. Ann. N. Y. Acad. Sci. 1476, 78–92 (2020).
Cote-L’Heureux, A., Maurer-Alcala, X. X. & Katz, L. A. Old genes in new places: a taxon-rich analysis of interdomain lateral gene transfer events. PLoS Genet. 18, e1010239 (2022).
Cummings, T. F. M. et al. Citrullination was introduced into animals by horizontal gene transfer from cyanobacteria. Mol. Biol. Evol. 39, msab317 (2022).
Katz, L. A. Recent events dominate interdomain lateral gene transfers between prokaryotes and eukaryotes and, with the exception of endosymbiotic gene transfers, few ancient transfer events persist. Philos. Trans. R. Soc. B 370, 20140324 (2015). This paper describes a systematic analysis of HGT across the tree of eukaryotes showing ancient transfers are rare in comparison with recent ones, mirroring earlier observations on bacteria.
Ku, C. et al. Endosymbiotic origin and differential loss of eukaryotic genes. Nature 524, 427–432 (2015).
Hao, W. & Golding, G. B. The fate of laterally transferred genes: life in the fast lane to adaptation or death. Genome Res. 16, 636–643 (2006).
Marri, P. R., Hao, W. & Golding, G. B. The role of laterally transferred genes in adaptive evolution. BMC Evol. Biol. 7, S8 (2007).
Eme, L., Gentekaki, E., Curtis, B., Archibald, J. M. & Roger, A. J. Lateral gene transfer in the adaptation of the anaerobic parasite Blastocystis to the gut. Curr. Biol. 27, 807–820 (2017).
Stairs, C. W. et al. Microbial eukaryotes have adapted to hypoxia by horizontal acquisitions of a gene involved in rhodoquinone biosynthesis. eLife 7, e34292 (2018).
Jimenez-Gonzalez, A., Xu, F. & Andersson, J. O. Lateral acquisitions repeatedly remodel the oxygen detoxification pathway in diplomonads and relatives. Genome Biol. Evol. 11, 2542–2556 (2019).
Murphy, C. L. et al. Horizontal gene transfer as an indispensable driver for evolution of neocallimastigomycota into a distinct gut-dwelling fungal lineage. Appl. Environ. Microbiol. 85, e00988-19 (2019).
Feng, J. M. et al. Single-cell transcriptome sequencing of rumen ciliates provides insight into their molecular adaptations to the anaerobic and carbohydrate-rich rumen microenvironment. Mol. Phylogenet. Evol. 143, 106687 (2020).
Lewis, W. H. et al. Convergent evolution of hydrogenosomes from mitochondria by gene transfer and loss. Mol. Biol. Evol. 37, 524–539 (2020).
Yubuki, N. et al. Ancient adaptive lateral gene transfers in the symbiotic Opalina–Blastocystis stramenopile lineage. Mol. Biol. Evol. 37, 651–659 (2020).
Žárský, V. et al. The Mastigamoeba balamuthi genome and the nature of the free-living ancestor of Entamoeba. Mol. Biol. Evol. 38, 2240–2259 (2021).
Woehle, C. et al. A novel eukaryotic denitrification pathway in foraminifera. Curr. Biol. 28, 2536–2543.e5 (2018).
Valach, M. et al. Recent expansion of metabolic versatility in Diplonema papillatum, the model species of a highly speciose group of marine eukaryotes. BMC Biol. 21, 99 (2023).
Schönknecht, G. et al. Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science 339, 1207–1210 (2013).
Striepen, B. et al. Gene transfer in the evolution of parasite nucleotide biosynthesis. Proc. Natl Acad. Sci. USA 101, 3154–3159 (2004).
Andersson, J. O. et al. A genomic survey of the fish parasite Spironucleus salmonicida indicates genomic plasticity among diplomonads and significant lateral gene transfer in eukaryote genome evolution. BMC Genom. 8, 51 (2007).
Haegeman, A., Jones, J. T. & Danchin, E. G. Horizontal gene transfer in nematodes: a catalyst for plant parasitism. Mol. Plant Microbe Interact. 24, 879–887 (2011).
Dean, P. et al. Transporter gene acquisition and innovation in the evolution of Microsporidia intracellular parasites. Nat. Commun. 9, 1709 (2018).
Sibbald, S. J., Eme, L., Archibald, J. M. & Roger, A. J. Lateral gene transfer mechanisms and pan-genomes in eukaryotes. Trends Parasitol. 36, 927–941 (2020).
Gilbert, C. & Maumus, F. Sidestepping Darwin: horizontal gene transfer from plants to insects. Curr. Opin. Insect Sci. 57, 101035 (2023).
Verster, K. I. et al. Evolution of insect innate immunity through domestication of bacterial toxins. Proc. Natl Acad. Sci. USA 120, e2218334120 (2023).
Walker, A. A. et al. Horizontal gene transfer underlies the painful stings of asp caterpillars (Lepidoptera: Megalopygidae). Proc. Natl Acad. Sci. USA 120, e2305871120 (2023).
Urquhart, A. S., Vogan, A. A., Gardiner, D. M. & Idnurm, A. Starships are active eukaryotic transposable elements mobilized by a new family of tyrosine recombinases. Proc. Natl Acad. Sci. USA 120, e2214521120 (2023). This study provides experimental evidence that eukaryotic transposable elements can move between species in fungi, with implications for accelerating the rates of HGT.
Douanne, N. et al. Leishmania parasites exchange drug-resistance genes through extracellular vesicles. Cell Rep. 40, 111121 (2022). This study provides experimental evidence that DNA can move between eukaryotic cells through extracellular vesicles, in this case leading to the spread of drug resistance in parasitic protists.
Marcilla, A. & Sanchez-Lopez, C. M. Extracellular vesicles as a horizontal gene transfer mechanism in Leishmania. Trends Parasitol. 38, 823–825 (2022).
Haimovich, G., Dasgupta, S. & Gerst, J. E. RNA transfer through tunneling nanotubes. Biochem. Soc. Trans. 49, 145–160 (2021).
Pereira, L., Christin, P. A. & Dunning, L. T. The mechanisms underpinning lateral gene transfer between grasses. Plants People Planet 5, 672–682 (2022).
Gilbert, C. & Cordaux, R. Viruses as vectors of horizontal transfer of genetic material in eukaryotes. Curr. Opin. Virol. 25, 16–22 (2017).
Malik, S. S., Azem-E-Zahra, S., Kim, K. M., Caetano-Anollés, G. & Nasir, A. Do viruses exchange genes across superkingdoms of life? Front. Microbiol. 8, 2110 (2017).
Irwin, N. A. T. et al. Viral proteins as a potential driver of histone depletion in dinoflagellates. Nat. Commun. 9, 1535 (2018).
Irwin, N. A. T., Pittis, A. A., Richards, T. A. & Keeling, P. J. Systematic evaluation of horizontal gene transfer between eukaryotes and viruses. Nat. Microbiol. 7, 327–336 (2022). This study describes the first systematic analysis of HGT between eukaryotes and their viruses, showing important functional impacts on both as well as confirming that transduction is a potentially major mechanism for HGT between eukaryotes.
Slamovits, C. H. & Keeling, P. J. Widespread recycling of processed cDNAs in dinoflagellates. Curr. Biol. 18, R550–R552 (2008).
Doolittle, W. F. You are what you eat: a gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes. Trends Genet. 14, 307–311 (1998).
Olofsson, J. K. et al. Population-specific selection on standing variation generated by lateral gene transfers in a grass. Curr. Biol. 29, 3921–3927.e5 (2019).
Thorsness, P. E. & Weber, E. R. Escape and migration of nucleic acids between chloroplasts, mitochondria, and the nucleus. Int. Rev. Cytol. 165, 207–234 (1996).
Huang, C. Y., Ayliffe, M. A. & Timmis, J. N. Direct measurement of the transfer rate of chloroplast DNA into the nucleus. Nature 422, 72–76 (2003).
Stegemann, S., Hartmann, S., Ruf, S. & Bock, R. High-frequency gene transfer from the chloroplast genome to the nucleus. Proc. Natl Acad. Sci. USA 100, 8828–8833 (2003).
Fuentes, I., Karcher, D. & Bock, R. Experimental reconstruction of the functional transfer of intron-containing plastid genes to the nucleus. Curr. Biol. 22, 763–771 (2012).
Matsuo, M., Ito, Y., Yamauchi, R. & Obokata, J. The rice nuclear genome continuously integrates, shuffles, and eliminates the chloroplast genome to cause chloroplast-nuclear DNA flux. Plant Cell 17, 665–675 (2005).
Dunning Hotopp, J. C. et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 317, 1753–1756 (2007).
Nikoh, N. et al. Wolbachia genome integrated in an insect chromosome: evolution and fate of laterally transferred endosymbiont genes. Genome Res. 18, 272–280 (2008).
Falkowski, P. G., Fenchel, T. & Delong, E. F. The microbial engines that drive Earth’s biogeochemical cycles. Science 320, 1034–1039 (2008).
Wright, J. J., Konwar, K. M. & Hallam, S. J. Microbial ecology of expanding oxygen minimum zones. Nat. Rev. Microbiol. 10, 381–394 (2012).
Louca, S., Parfrey, L. W. & Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 353, 1272–1277 (2016).
Doolittle, W. F. Darwinizing Gaia. J. Theor. Biol. 434, 11–19 (2017).
Glasner, M. E., Truong, D. P. & Morse, B. C. How enzyme promiscuity and horizontal gene transfer contribute to metabolic innovation. FEBS J. 287, 1323–1342 (2020).
Keeling, P. J. Combining morphology, behaviour and genomics to understand the evolution and ecology of microbial eukaryotes. Philos. Trans. R. Soc. B 374, 20190085 (2019).
Richards, T. A., Leonard, G., Soanes, D. & Talbot, N. J. Gene transfer into the fungi. Fungal Biol. Rev. 25, 98–110 (2011).
Slot, J. C. & Rokas, A. Horizontal transfer of a large and highly toxic secondary metabolic gene cluster between fungi. Curr. Biol. 21, 134–139 (2011).
Fitzpatrick, D. A. Horizontal gene transfer in fungi. FEMS Microbiol. Lett. 329, 1–8 (2012).
Shen, X. X. et al. Tempo and mode of genome evolution in the budding yeast subphylum. Cell 175, 1533–1545 e1520 (2018).
Slot, J. C. & Hibbett, D. S. Horizontal transfer of a nitrate assimilation gene cluster and ecological transitions in fungi: a phylogenetic study. PLoS ONE 2, e1097 (2007).
Richards, T. A. Genome evolution: horizontal movements in the fungi. Curr. Biol. 21, R166–R168 (2011).
Milner, D. S. et al. Environment-dependent fitness gains can be driven by horizontal gene transfer of transporter-encoding genes. Proc. Natl Acad. Sci. USA 116, 5613–5622 (2019). Transporters are a classes of proteins that might confer a strong and immediate selective advantage, and this work shows they spread among eukaryotes by HGT.
Ocana-Pallares, E. et al. Divergent genomic trajectories predate the origin of animals and fungi. Nature 609, 747–753 (2022).
Soanes, D. M., Richards, T. A. & Talbot, N. J. Insights from sequencing fungal and oomycete genomes: what can we learn about plant disease and the evolution of pathogenicity? Plant Cell 19, 3318–3326 (2007).
Richards, T. A. et al. Horizontal gene transfer facilitated the evolution of plant parasitic mechanisms in the oomycetes. Proc. Natl Acad. Sci. USA 108, 15258–15263 (2011).
Richards, T. A. & Talbot, N. J. Horizontal gene transfer in osmotrophs: playing with public goods. Nat. Rev. Microbiol. 11, 720–727 (2013).
Savory, F., Leonard, G. & Richards, T. A. The role of horizontal gene transfer in the evolution of the oomycetes. PLoS Pathog. 11, e1004805 (2015).
Savory, F. R., Milner, D. S., Miles, D. C. & Richards, T. A. Ancestral function and diversification of a horizontally acquired oomycete carboxylic acid transporter. Mol. Biol. Evol. 35, 1887–1900 (2018).
Richardson, A. O. & Palmer, J. D. Horizontal gene transfer in plants. J. Exp. Bot. 58, 1–9 (2007).
Gao, C. et al. Horizontal gene transfer in plants. Funct. Integr. Genomics 14, 23–29 (2014).
Davis, C. C. & Xi, Z. Horizontal gene transfer in parasitic plants. Curr. Opin. Plant Biol. 26, 14–19 (2015).
Dunning, L. T. & Christin, P. A. Reticulate evolution, lateral gene transfer, and innovation in plants. Am. J. Bot. 107, 541–544 (2020).
Wickell, D. A. & Li, F. W. On the evolutionary significance of horizontal gene transfers in plants. New Phytol. 225, 113–117 (2020).
Aubin, E., El Baidouri, M. & Panaud, O. Horizontal gene transfers in plants. Life 11, 857 (2021).
Cho, Y., Qiu, Y. L., Kuhlman, P. & Palmer, J. D. Explosive invasion of plant mitochondria by a group I intron. Proc. Natl Acad. Sci. USA 95, 14244–14249 (1998).
Cho, Y. & Palmer, J. D. Multiple acquisitions via horizontal transfer of a group I intron in the mitochondrial cox1 gene during evolution of the Araceae family. Mol. Biol. Evol. 16, 1155–1165 (1999).
Palmer, J. D. et al. Dynamic evolution of plant mitochondrial genomes: mobile genes and introns and highly variable mutation rates. Proc. Natl Acad. Sci. USA 97, 6960–6966 (2000).
Sinn, B. T. & Barrett, C. F. Ancient mitochondrial gene transfer between fungi and the orchids. Mol. Biol. Evol. 37, 44–57 (2020).
Garcia, L. E., Edera, A. A., Palmer, J. D., Sato, H. & Sanchez-Puerta, M. V. Horizontal gene transfers dominate the functional mitochondrial gene space of a holoparasitic plant. New Phytol. 229, 1701–1714 (2021).
Yue, J., Hu, X., Sun, H., Yang, Y. & Huang, J. Widespread impact of horizontal gene transfer on plant colonization of land. Nat. Commun. 3, 1152 (2012).
Cheng, S. et al. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell 179, 1057–1067.e1014 (2019).
Chen, R. et al. Adaptive innovation of green plants by horizontal gene transfer. Biotechnol. Adv. 46, 107671 (2021).
Won, H. & Renner, S. S. Horizontal gene transfer from flowering plants to Gnetum. Proc. Natl Acad. Sci. USA 100, 10824–10829 (2003).
Davis, C. C., Anderson, W. R. & Wurdack, K. J. Gene transfer from a parasitic flowering plant to a fern. Proc. Biol. Sci. 272, 2237–2242 (2005).
Kado, T. & Innan, H. Horizontal gene transfer in five parasite plant species in Orobanchaceae. Genome Biol. Evol. 10, 3196–3210 (2018).
Dunning, L. T. et al. Lateral transfers of large DNA fragments spread functional genes among grasses. Proc. Natl Acad. Sci. USA 116, 4416–4425 (2019).
Phansopa, C., Dunning, L. T., Reid, J. D. & Christin, P. A. Lateral gene transfer acts as an evolutionary shortcut to efficient C4 biochemistry. Mol. Biol. Evol. 37, 3094–3104 (2020). This paper shows that the diversity of a key enzyme in C4 photosynthesis in a grass species comes from a combination of mutation and HGT in different populations, and those populations in which HGT plays a role adopt advantageous enzyme kinetics more rapidly.
Hibdige, S. G. S., Raimondeau, P., Christin, P. A. & Dunning, L. T. Widespread lateral gene transfer among grasses. New Phytol. 230, 2474–2486 (2021). This paper describes a systemic analysis of HGT in grasses, including important crops, showing both prevalence and trends across the group that help outline potential mechanisms and functional outcomes.
Ma, J. et al. Major episodes of horizontal gene transfer drove the evolution of land plants. Mol. Plant 15, 857–871 (2022). This paper describes a comprehensive analysis of HGT in plants linking gains of functionally important genes to major transitions in plant evolution.
Prasad, A., Chirom, O. & Prasad, M. Horizontal gene transfer and the evolution of land plants. Trends Plant Sci. 27, 1203–1205 (2022).
Royo, J., Gimez, E. & Hueros, G. CMP–KDO synthetase: a plant gene borrowed from gram-negative eubacteria. Trends Genet. 16, 432–433 (2000).
Yue, J., Hu, X. & Huang, J. Origin of plant auxin biosynthesis. Trends Plant Sci. 19, 764–770 (2014).
Yang, Z. et al. Ancient horizontal transfer of transaldolase-like protein gene and its role in plant vascular development. New Phytol. 206, 807–816 (2015).
Wang, Q., Smith, S. M. & Huang, J. Origins of strigolactone and karrikin signaling in plants. Trends Plant Sci. 27, 450–459 (2022).
Davis, C. C. & Wurdack, K. J. Host-to-parasite gene transfer in flowering plants: phylogenetic evidence from Malpighiales. Science 305, 676–678 (2004).
Barkman, T. J. et al. Mitochondrial DNA suggests at least 11 origins of parasitism in angiosperms and reveals genomic chimerism in parasitic plants. BMC Evol. Biol. 7, 248 (2007).
Vogel, A. et al. Footprints of parasitism in the genome of the parasitic flowering plant Cuscuta campestris. Nat. Commun. 9, 2515 (2018).
Cusimano, N. & Renner, S. S. Sequential horizontal gene transfers from different hosts in a widespread Eurasian parasitic plant, Cynomorium coccineum. Am. J. Bot. 106, 679–689 (2019).
Cai, L. et al. Deeply altered genome architecture in the endoparasitic flowering plant Sapria himalayana griff. (Rafflesiaceae). Curr. Biol. 31, 1002–1011.e9 (2021).
Westwood, J. H. Plant biology: genome reveals secrets of the alien within. Curr. Biol. 31, R241–R243 (2021).
Guo, X. et al. The Sapria himalayana genome provides new insights into the lifestyle of endoparasitic plants. BMC Biol. 21, 134 (2023).
Bonen, L., Cunningham, R. S., Gray, M. W. & Doolittle, W. F. Wheat embryo mitochondrial 18 S ribosomal RNA: evidence for its prokaryotic nature. Nucleic Acids Res. 4, 663–671 (1977).
Yang, D., Oyaizu, Y., Oyaizu, H., Olsen, G. J. & Woese, C. R. Mitochondrial origins. Proc. Natl Acad. Sci. USA 82, 4443–4447 (1985).
Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 14, 225–274 (1967).
Goksøyr, J. Evolution of eucaryotic cells. Nature 214, 1161 (1967).
Weeden, N. F. Genetic and biochemical implications of the endosymbiotic origin of the chloroplast. J. Mol. Evol. 17, 133–139 (1981).
Adams, K. L., Daley, D. O., Qiu, Y. L., Whelan, J. & Palmer, J. D. Repeated, recent and diverse transfers of a mitochondrial gene to the nucleus in flowering plants. Nature 408, 354–357 (2000).
Millen, R. S. et al. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell 13, 645–658 (2001).
Adams, K. L., Qiu, Y. L., Stoutemyer, M. & Palmer, J. D. Punctuated evolution of mitochondrial gene content: high and variable rates of mitochondrial gene loss and transfer to the nucleus during angiosperm evolution. Proc. Natl Acad. Sci. USA 99, 9905–9912 (2002).
van Dooren, G. G., Su, V., D’Ombrain, M. C. & McFadden, G. I. Processing of an apicoplast leader sequence in Plasmodium falciparum and the identification of a putative leader cleavage enzyme. J. Biol. Chem. 277, 23612–23619 (2002).
Choi, C., Liu, Z. & Adams, K. L. Evolutionary transfers of mitochondrial genes to the nucleus in the Populus lineage and coexpression of nuclear and mitochondrial Sdh4 genes. New Phytol. 172, 429–439 (2006).
Bock, R. & Timmis, J. N. Reconstructing evolution: gene transfer from plastids to the nucleus. Bioessays 30, 556–566 (2008).
Bock, R. Witnessing genome evolution: experimental reconstruction of endosymbiotic and horizontal gene transfer. Annu. Rev. Genet. 51, 1–22 (2017).
Martin, W. Evolutionary origins of metabolic compartmentalization in eukaryotes. Philos. Trans. R. Soc. B 365, 847–855 (2010).
Martin, W. et al. Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc. Natl Acad. Sci. USA 99, 12246–12251 (2002).
Palenik, B. The genomics of symbiosis: hosts keep the baby and the bath water. Proc. Natl Acad. Sci. USA 99, 11996–11997 (2002).
Gabaldon, T. & Huynen, M. A. Reconstruction of the proto-mitochondrial metabolism. Science 301, 609 (2003).
Hannaert, V. et al. Plant-like traits associated with metabolism of Trypanosoma parasites. Proc. Natl Acad. Sci. USA 100, 1067–1071 (2003).
Reyes-Prieto, A., Moustafa, A. & Bhattacharya, D. Multiple genes of apparent algal origin suggest ciliates may once have been photosynthetic. Curr. Biol. 18, 956–962 (2008).
Moustafa, A. et al. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 324, 1724–1726 (2009).
Woehle, C., Dagan, T., Martin, W. F. & Gould, S. B. Red and problematic green phylogenetic signals among thousands of nuclear genes from the photosynthetic and apicomplexa-related Chromera velia. Genome Biol. Evol. 3, 1220–1230 (2011).
Patron, N. J., Waller, R. F. & Keeling, P. J. A tertiary plastid uses genes from two endosymbionts. J. Mol. Biol. 357, 1373–1382 (2006).
Reyes-Prieto, A., Hackett, J. D., Soares, M. B., Bonaldo, M. F. & Bhattacharya, D. Cyanobacterial contribution to algal nuclear genomes is primarily limited to plastid functions. Curr. Biol. 16, 2320–2325 (2006).
Curtis, B. A. et al. Algal genomes reveal evolutionary mosaicism and the fate of nucleomorphs. Nature 492, 59–65 (2012).
Burki, F. et al. Endosymbiotic gene transfer in tertiary plastid-containing dinoflagellates. Eukaryot. Cell 13, 246–255 (2014).
Hehenberger, E., Gast, R. J. & Keeling, P. J. A kleptoplastidic dinoflagellate and the tipping point between transient and fully integrated plastid endosymbiosis. Proc. Natl Acad. Sci. USA 116, 17934–17942 (2019). This paper, and Karnkowska et al. (2023), analyse plastid-targeted proteins from kleptoplastic sisters of lineages with secondary or tertiary plastids, and both show the presence of hetero-EPT and that protein targeting evolved before the organelle was permanently integrated.
Burki, F. et al. Re-evaluating the green versus red signal in eukaryotes with secondary plastid of red algal origin. Genome Biol. Evol. 4, 626–635 (2012).
Deschamps, P. & Moreira, D. Reevaluating the green contribution to diatom genomes. Genome Biol. Evol. 4, 683–688 (2012).
Moreira, D. & Deschamps, P. What was the real contribution of endosymbionts to the eukaryotic nucleus? Insights from photosynthetic eukaryotes. Cold Spring Harb. Perspect. Biol. 6, a016014 (2014).
Karnkowska, A. et al. A eukaryote without a mitochondrial organelle. Curr. Biol. 26, 1274–1284 (2016).
Gornik, S. G. et al. Endosymbiosis undone by stepwise elimination of the plastid in a parasitic dinoflagellate. Proc. Natl Acad. Sci. USA 112, 5767–5772 (2015). This paper describes one of the earliest cases of complete loss of an organelle (the plastid in this case) that also showed no evidence for relict organelle-derived genes (that is, no hetero-EGT).
Mathur, V. et al. Multiple independent origins of apicomplexan-like parasites. Curr. Biol. 29, 2936–2941.e5 (2019).
Schön, M. E. et al. Single cell genomics reveals plastid-lacking Picozoa are close relatives of red algae. Nat. Commun. 12, 6651 (2021).
Gawryluk, R. M. R. et al. Non-photosynthetic predators are sister to red algae. Nature 572, 240–243 (2019).
Archibald, J. M., Rogers, M. B., Toop, M., Ishida, K. & Keeling, P. J. Lateral gene transfer and the evolution of plastid-targeted proteins in the secondary plastid-containing alga Bigelowiella natans. Proc. Natl Acad. Sci. USA 100, 7678–7683 (2003).
Suzuki, K. & Miyagishima, S. Y. Eukaryotic and eubacterial contributions to the establishment of plastid proteome estimated by large-scale phylogenetic analyses. Mol. Biol. Evol. 27, 581–590 (2010).
Szklarczyk, R. & Huynen, M. A. Mosaic origin of the mitochondrial proteome. Proteomics 10, 4012–4024 (2010).
Qiu, H. et al. Assessing the bacterial contribution to the plastid proteome. Trends Plant Sci. 18, 680–687 (2013).
Gray, M. W. Mosaic nature of the mitochondrial proteome: implications for the origin and evolution of mitochondria. Proc. Natl Acad. Sci. USA 112, 10133–10138 (2015).
Marin, B., Nowack, E. C. & Melkonian, M. A plastid in the making: evidence for a second primary endosymbiosis. Protist 156, 425–432 (2005).
Nowack, E. C. M., Melkonian, M. & Glöckner, G. Chromatophore genome sequence of Paulinella sheds light on acquisition of photosynthesis by eukaryotes. Curr. Biol. 18, 410–418 (2008).
Nowack, E. C. & Grossman, A. R. Trafficking of protein into the recently established photosynthetic organelles of Paulinella chromatophora. Proc. Natl Acad. Sci. USA 109, 5340–5345 (2012). This paper was among the first to provide evidence for protein-targeting to an endosymbiont other than the mitochondria or plastids, an important step widely believed to mark the distinguishing characteristic of an organelle.
Nowack, E. C. et al. Gene transfers from diverse bacteria compensate for reductive genome evolution in the chromatophore of Paulinella chromatophora. Proc. Natl Acad. Sci. USA 113, 12214–12219 (2016).
Singer, A. et al. Massive protein import into the early-evolutionary-stage photosynthetic organelle of the amoeba Paulinella chromatophora. Curr. Biol. 27, 2763–2773.e5 (2017). This paper describes a comprehensive analysis of the proteins targeted to the chromatophore showing that many are not derived from the same lineage as the organelle itself.
Nowack, E. C. M. & Weber, A. P. M. Genomics-informed insights into endosymbiotic organelle evolution in photosynthetic eukaryotes. Annu. Rev. Plant Biol. 69, 51–84 (2018).
Oberleitner, L., Perrar, A., Macorano, L., Huesgen, P. F. & Nowack, E. C. M. A bipartite chromatophore transit peptide and N-terminal protein processing in the Paulinella chromatophore. Plant Physiol. 189, 152–164 (2022).
Morales, J. et al. Host-symbiont interactions in Angomonas deanei include the evolution of a host-derived dynamin ring around the endosymbiont division site. Curr. Biol. 33, 28–40.e7 (2023).
Dyall, S. D., Brown, M. T. & Johnson, P. J. Ancient invasions: from endosymbionts to organelles. Science 304, 253–257 (2004).
Karnkowska, A. et al. Euglenozoan kleptoplasty illuminates the early evolution of photoendosymbiosis. Proc. Natl Acad. Sci. USA 120, e2220100120 (2023). This paper, and Hehenberger et al., analyse plastid-targeted proteins from kleptoplastic sisters of lineages with secondary or tertiary plastids, and both show the presence of hetero-EPT and that protein targeting evolved before the organelle was permanently integrated.
Larkum, A. W., Lockhart, P. J. & Howe, C. J. Shopping for plastids. Trends Plant Sci. 12, 189–195 (2007).
Keeling, P. J. The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu. Rev. Plant Biol. 64, 583–607 (2013).
Zachar, I., Szilagyi, A., Szamado, S. & Szathmary, E. Farming the mitochondrial ancestor as a model of endosymbiotic establishment by natural selection. Proc. Natl Acad. Sci. USA 115, E1504–E1510 (2018).
Keeling, P. J. & Burki, F. Progress towards the tree of eukaryotes. Curr. Biol. 29, R808–R817 (2019).
Crisp, A., Boschetti, C., Perry, M., Tunnacliffe, A. & Micklem, G. Expression of multiple horizontally acquired genes is a hallmark of both vertebrate and invertebrate genomes. Genome Biol. 16, 50 (2015).
Salzberg, S. L. & Eisen, J. A. Lateral gene transfer or viral colonization? Science 293, 1048 (2001).
Salzberg, S. L. Horizontal gene transfer is not a hallmark of the human genome. Genome Biol. 18, 85 (2017).
Arakawa, K. No evidence for extensive horizontal gene transfer from the draft genome of a tardigrade. Proc. Natl Acad. Sci. USA 113, E3057 (2016).
Bemm, F., Weiss, C. L., Schultz, J. & Forster, F. Genome of a tardigrade: horizontal gene transfer or bacterial contamination? Proc. Natl Acad. Sci. USA 113, E3054–E3056 (2016).
Koutsovoulos, G. et al. No evidence for extensive horizontal gene transfer in the genome of the tardigrade Hypsibius dujardini. Proc. Natl Acad. Sci. USA 113, 5053–5058 (2016).
Gladyshev, E. A., Meselson, M. & Arkhipova, I. R. Massive horizontal gene transfer in bdelloid rotifers. Science 320, 1210–1213 (2008). High frequencies of HGTs to animals had been erroneously reported previously, but this case reporting many HGTs in bdelloid rotifers has withstood comprehensive reanalysis, making these rotifers unique among metazoans.
Eyres, I. et al. Horizontal gene transfer in bdelloid rotifers is ancient, ongoing and more frequent in species from desiccating habitats. BMC Biol. 13, 90 (2015).
Nowell, R. W. et al. Comparative genomics of bdelloid rotifers: insights from desiccating and nondesiccating species. PLoS Biol. 16, e2004830 (2018).
Gladyshev, E. A. & Arkhipova, I. R. Genome structure of bdelloid rotifers: shaped by asexuality or desiccation? J. Hered. 101, S85–S93 (2010).
Debortoli, N. et al. Genetic exchange among bdelloid rotifers is more likely due to horizontal gene transfer than to meiotic sex. Curr. Biol. 26, 723–732 (2016).
Signorovitch, A., Hur, J., Gladyshev, E. & Meselson, M. Evidence for meiotic sex in bdelloid rotifers. Curr. Biol. 26, R754–R755 (2016).
Li, X. et al. Desiccation does not drastically increase the accessibility of exogenous DNA to nuclear genomes: evidence from the frequency of endosymbiotic DNA transfer. BMC Genom. 21, 452 (2020).
Acknowledgements
The author thanks F. Doolittle, J. McCutcheon, V. Boscaro and N. Irwin for a number of long and interesting discussions on the topic of HGT in general; F. Burki, V. Boscaro and L. Hehenberger for debate over EGT terminology; and N. Irwin, N. Fast and T. Richards for their critical reading of the manuscript. This work was supported by a grant from the Gordon and Betty Moore Foundation (https://doi.org/10.37807/GBMF9201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Laura Eme, Ewa Nowack and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keeling, P.J. Horizontal gene transfer in eukaryotes: aligning theory with data. Nat Rev Genet (2024). https://doi.org/10.1038/s41576-023-00688-5
Accepted:
Published:
DOI: https://doi.org/10.1038/s41576-023-00688-5