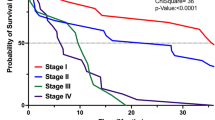
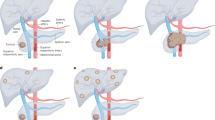
Abstract
Surgical resection combined with systemic chemotherapy is the cornerstone of treatment for patients with localized pancreatic cancer. Upfront surgery is considered suboptimal in cases with extensive vascular involvement, which can be classified as either borderline resectable pancreatic cancer or locally advanced pancreatic cancer. In these patients, FOLFIRINOX or gemcitabine plus nab-paclitaxel chemotherapy is currently used as preoperative chemotherapy and is eventually combined with radiotherapy. Thus, more patients might reach 5-year overall survival. Patient selection for chemotherapy, radiotherapy and subsequent surgery is based on anatomical, biological and conditional parameters. Current guidelines and clinical practices vary considerably regarding preoperative chemotherapy and radiotherapy, response evaluation, and indications for surgery. In this Review, we provide an overview of the clinical evidence regarding disease staging, preoperative therapy, response evaluation and surgery in patients with borderline resectable pancreatic cancer or locally advanced pancreatic cancer. In addition, a clinical work-up is proposed based on the available evidence and guidelines. We identify knowledge gaps and outline a proposed research agenda.
Key points
-
Preoperative multi-agent chemotherapy (for example, FOLFIRINOX or gemcitabine plus nab-paclitaxel) is now routinely used in patients with borderline resectable pancreatic cancer (BRPC) or locally advanced pancreatic cancer (LAPC), both to obtain local and systemic control and to select suitable candidates for surgery.
-
Considerable variation exists among national and international guidelines and clinical practices regarding preoperative therapy in patients with BRPC or LAPC, including the type and duration of chemotherapy and the role, type, and timing of radiotherapy; a uniform, evidence-based international guideline with support from all relevant societies is needed.
-
Three randomized controlled trials reported improved outcomes with neoadjuvant chemotherapy or chemoradiotherapy compared with upfront surgery in patients with BRPC; more randomized trials assessing the effect of modern multi-agent chemotherapy and radiotherapy are needed and several are ongoing.
-
Response evaluation after preoperative chemotherapy and chemoradiotherapy is a major challenge as conventional cross-sectional imaging mostly underestimates the tumour response. Biological response evaluation is therefore advised (particularly a relative decrease of serum CA19-9). However, there is an urgent need for more accurate tumour markers.
-
Surgery after preoperative therapy in patients with BRPC and LAPC requires high-volume expertise for patient selection, intraoperative decision-making, extended resections and postoperative care; preoperative counselling and shared decision-making are crucial.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel, R. L. et al. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Rahib, L. et al. Estimated projection of US cancer incidence and death to 2040. JAMA Netw. Open. 4, e214708 (2021).
Mizrahi, J. D., Surana, R., Valle, J. W. & Shroff, R. T. et al. Pancreatic cancer. Lancet 395, 2008–2020 (2020).
Springfeld, C. et al. Neoadjuvant therapy for pancreatic cancer. Nat. Rev. Clin. Oncol. 20, 318–337 (2023).
Strobel, O. et al. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 16, 11–26 (2019).
Groot, V. P. et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann. Surg. 267, 936–945 (2018).
Daamen, L. A. et al. Preoperative predictors for early and very early disease recurrence in patients undergoing resection of pancreatic ductal adenocarcinoma. HPB 24, 535–546 (2022).
Seelen, L. W. F. et al. Early recurrence after resection of locally advanced pancreatic cancer following induction therapy: an international multicenter study. Ann. Surg. 278, 118–126 (2023).
Neoptolemos, J. P. et al. Comparison of adjuvant gemcitabine and capecitrabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicenter, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024 (2017).
Conroy, T. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379, 2395–2406 (2018).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Muller, P. C. et al. Neoadjuvant chemotherapy in pancreatic cancer: an appraisal of the current high-level evidence. Pharmacology 106, 143–153 (2020).
Brown, Z. J. & Cloyd, J. M. Trends in the utilization of neoadjuvant therapy for pancreatic ductal adenocarcinoma. J. Surg. Oncol. 123, 1432–1440 (2021).
Oba, A. et al. Neoadjuvant treatment in pancreatic cancer. Front. Oncol. 10, 245 (2020).
Ratnayake, B. et al. Recurrence patterns for pancreatic ductal adenocarcinoma after upfront resection versus resection following neoadjuvant therapy: a comprehensive meta-analysis. J. Clin. Med. 9, 2132 (2020).
Sohal, D. P., Walsh, R. M., Ramanathan, R. K. & Khorana, A. A. Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J. Natl Cancer Inst. 106, dju011 (2014).
Heinrich, S. et al. Opinions and use of neoadjuvant therapy for resectable, borderline resectable, and locally advanced pancreatic cancer: international survey and case-vignette study. BMC Cancer 19, 675 (2019).
Reames, B. N. et al. Management of locally advanced pancreatic cancer: results of an international survey of current practice. Ann. Surg. 273, 1173–1181 (2021).
Khachfe, H. H., Habib, J. R., Nassour, I., Al Harthi, S. & Jamali, F. R. Borderline resectable and locally advanced pancreatic cancers: a review of definitions, diagnostics, strategies for treatment, and future directions. Pancreas 50, 1243–1249 (2021).
Takaori, K. et al. International Association of Pancreatology (IAP)/European Pancreatic Club (EPC) consensus review of guidelines for the treatment of pancreatic cancer. Pancreatology 16, 14–27 (2016).
Tempero, M. A. et al. Pancreatic Adenocarcinoma, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network https://www.nccn.org/home (2022).
Vauthey, J. N. & Dixon, E. AHPBA/SSO/SSAT consensus conference on resectable and borderline resectable pancreatic cancer: rationale and overview of the conference. Ann. Surg. Oncol. 16, 1725–1726 (2009).
Das, P. et al. Pancreatic Adenocarcinoma. MD Anderson Cancer Center https://www.mdanderson.org/content/dam/mdanderson/documents/for-physicians/algorithms/cancer-treatment/ca-treatment-pancreatic-web-algorithm.pdf (2021).
Katz, M. H. G. et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann. Surg. Oncol. 20, 2787–2795 (2013).
Japan Pancreas Society. General Rules for the Study of Pancreatic Cancer 7th edn (Kanehara & Co., Ltd, 2016).
Lutz, M. P. et al. Gallen EORTC gastrointestinal cancer conference: consensus recommendations on controversial issues in the primary tratment of pancreatic cancer. Eur. J. Cancer 79, 41–49 (2017).
Wu, Y. H. A. et al. Selecting surgical candidates with locally advanced pancreatic cancer: a review for modern pancreatology. J. Gastrointest. Oncol. 12, 2475–2483 (2021).
Bratlie, S. O., Wennerblom, J., Vilhav, C., Persson, J. & Rangelova, E. Resectable, borderline, and locally advanced pancreatic cancer — “the good, the bad, and the ugly” candidates for surgery? J. Gastrointest. Oncol. 12, 2450–2460 (2021).
Habib, J. R. & Wolfgang, C. L. Commentary: anatomic versus biologic resectability: the role of predictive biomarkers in guiding surgical management. Surgery 168, 1017–1018 (2020).
Khorana, A. A. et al. Potentially curable pancreatic cancer: american society of clinical oncology clinical practice guideline. J. Clin. Oncol. 34, 2541–2556 (2016).
Khorana, A. A. et al. Potentially curable pancreatic adenocaricnoma: ASCO clinical practice guideline update. J. Clin. Oncol. 37, 2082–2088 (2019).
Balaban, E. P. et al. Locally advanced, unresectable pancreatic cancer: american society of clinical oncology clinical practice guideline. J. Clin. Oncol. 34, 2654–2668 (2016).
Ducreux, M. et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26, v56–v68 (2015).
Pentheroudakis, G., ESMO Guidelines Committee. Recent eUpdates to the ESMO Clinical Practice Guidelines on hepatocellular carcinoma, cancer of the pancreas, soft tissue and visceral sarcomas, cancer of the prostate and gastric cancer. Ann. Oncol. 30, 1395–1397 (2019).
Neuzillet, C. et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig. Liver Dis. 50, 1257–1271 (2018).
Katz, M. H. G. et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J. Am. Coll. Surg. 206, 833–848 (2008).
Isaji, S. et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 18, 2–11 (2018).
Anger, F. et al. Impact of borderline resectability in pancreatic head cancer on patient survival: biology matters according to the new international consensus criteria. Ann. Surg. Oncol. 28, 2325–2336 (2021).
Tzeng, C. W. et al. Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J. Gastrointest. Surg. 18, 146–156 (2014).
Tsai, S. et al. Multimodality therapy in patients with borderline resectable or locally advanced pancreatic cancer: importance of locoregional therapies for a systemic disease. J. Oncol. Pract. 12, 915–923 (2016).
Chatzizacharias, N. A. et al. Locally advanced pancreas cancer: staging and goals of therapy. Surgery 163, 1053–1062 (2018).
Fromer, M. W. et al. An improved staging system for locally advanced pancreatic cancer: a critical need in the multidisciplinary era. Ann. Surg. Oncol. 28, 6201–6210 (2021).
Inoue, Y. et al. Radical resection for locally advanced pancreatic cancers in the era of new neoadjuvant therapy - arterial resection, arterial divestment and total pancreatectomy. Cancers 13, 1818 (2021).
Gemenetzis, G. et al. Anatomic criteria determine resectability in locally advanced pancreatic cancer. Ann. Surg. Oncol. 29, 401–414 (2022).
Vernerey, D. et al. Prognostic nomogram and score to predict overall survival in locally advanced untreated pancreatic cancer (PROLAP). Br. J. Cancer 115, 281–289 (2016).
Choi, S. H., Park, S. W. & Seong, J. A nomogram for predicting survival of patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Radiother. Oncol. 129, 340–346 (2018).
Ishii, N. et al. Proposal of predictive model on survival in unresectable pancreatic cancer receiving systemic chemotherapy. J. Cancer 11, 1223–1230 (2020).
Tong, Z. et al. Development, validation and comparison of artificial neural network models and logistic regression models predicting survival of unresectable pancreatic cancer. Front. Bioeng. Biotechnol. 8, 196 (2020).
Hwang, H. K. et al. A nomogram to preoperatively predict 1-year disease-specific survival in resected pancreatic cancer following neoadjuvant chemoradiation therapy. Chin. J. Cancer Res. 32, 105–114 (2020).
Brada, L. J. H. et al. Pedicting overall survival and resection in patients with locally advanced pancreatic cancer treated with FOLFIRINOX: development and internal validation of two nomograms. J. Surg. Oncol. 124, 589–597 (2021).
Zhu, X. et al. Development and validation of multicenter predictive nomograms for locally advanced pancreatic cancer after chemoradiotherapy. Front. Oncol. 11, 688576 (2021).
Ren, W., Xourafas, D., Ashley, S. W. & Clancy, T. E. Predicting surgical margins in patients with borderline resectable and locally advanced pancreatic cancer undergoing resection. Am. Surg. 88, 2899–2906 (2022).
Oba, A. et al. Prognosis based definition of resectability in pancreatic cancer: a road map to new guidelines. Ann. Surg. 275, 175–181 (2022).
Shibuki, T. et al. Prognostic nomogram for patients with unresectable pancreatic cancer treated with gemcitabine plus nab-paclitaxel or FOLFIRINOX: a post-hoc analysis of a multicenter retrospective study in Japan (NAPOLEON study). BMC Cancer 22, 19 (2022).
Habib, J. R. et al. Surgical decision making in pancreatic ductal adenocarcinoma: modeling prognosis following pancreatectomy in the era of induction and neoadjuvant chemotherapy. Ann. Surg. 277, 151–158 (2023).
Lu, Z. et al. Identifying optimal candidates for tumor resection among borderline and locally advanced pancreatic cancer: a population-based predictive model. Pancreatology 22, 286–293 (2022).
Tanaka, M. et al. Induction chemotherapy with FOLFIRINOX for locally advanced pancreatic cancer: a simple scoring system to predict effect and prognosis. Ann. Surg. Oncol. 30, 2401–2408 (2022).
Janssen, Q. P. et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J. Natl Cancer Inst. 111, 782–794 (2019).
Damm, M. et al. Efficacy and safety of neoadjuvant gemcitabine plus nab-paclitaxel in borderline resectable and locally advanced pancreatic cancer — a systematic review and meta-analysis. Cancers 13, 4326 (2021).
Jang, J. Y. et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann. Surg. 268, 215–222 (2018).
Versteijne, E. et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 38, 1763–1773 (2020).
Versteijne, E. et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the dutch randomized PREOPANC trial. J. Clin. Oncol. 40, 1220–1230 (2022).
Ghaneh, P. et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 8, 157–168 (2023).
Yamaguchi, J. et al. Results of a phase II study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab-PTX) for borderline-resectable pancreatic cancer (NUPAT-01). Ann. Surg. 275, 1043–1049 (2022).
Oar, A. et al. AGITG MASTERPLAN: a randomised phase II study of modified FOLFIRINOX alone or in combination with stereotactic body radiotherapy for patients with high-risk and locally advanced pancreatic cancer. BMC Cancer 21, 936 (2021).
World Health Organization. Effect of Stereotactic Ablative Body Radiotherapy for Unresectable Pancreatic Cancer with Endoscopic Ultrasound Inserted Fiducial Markers and Concurrent Chemotherapy on Survival Rates: SUPER Trial. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12617001571369 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05083247 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02676349 (2023).
World Health Organization. The Utility of Preoperative Chemoradiotherapy (Gem/nab-PTX+RT) and Chemotherapy (Gem/nab-PTX) for Borderline Resectable Pancreatic Cancer: Multicenter Randomized Phase II Trial — CSGO-HBP-021. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-jRCTs051200130 (2021).
World Health Organization. Phase II Study of Neoadjuvant FOLFIRINOX or Nab-Paclitaxel With Gemcitabine for Borderline Resectable Pancreatic Cancer — Phase II Study of Neoadjuvant FOLFIRINOX or Nab-Paclitaxel With Gemcitabine for Borderline Resectable Pancreatic Cancer. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000017718 (2015).
World Health Organization. Clinical Study of Neoadjuvant Therapy Used in Borderline Resectable Pancreatic Cancer. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-INR-17012555 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04617821 (2022).
Janssen, Q. P. et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer 21, 300 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04793932 (2021).
Cascinu, S. et al. Nab-paclitaxel/gemcitabine combination is more effective than gemcitabine alone in locally advanced, unresectable pancreatic cancer — a GISCAD phase II randomized trial. Eur. J. Cancer 148, 422–429 (2021).
Su, Y. Y. et al. A phase II randomised trial of induction chemotherapy followed by concurrent chemoradiotherapy in locally advanced pancreatic cancer: the Taiwan Cooperative Oncology Group T2212 study. Br. J. Cancer 126, 1018–1026 (2022).
Dehbi, H. M. & Hackshaw, A. Sample size calculation in randomised phase II selection trials using a margin of practical equivalence. Trials 21, 301 (2020).
Ozaka, M. et al. A randomised phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel for locally advanced pancreatic cancer (JCOG1407). Eur. J. Cancer 181, 135–144 (2022).
Suker, M. et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 17, 801–810 (2016).
Chen, Z. et al. Meta-analysis of FOLFIRINOX-based neoadjuvant therapy for locally advanced pancreatic cancer. Medicine 100, e24068 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04986930 (2023).
World Health Organization. A Randomized Phase III Study Evaluating Modified FOLFIRINOX (mFFX) With or Without Stereotactic Body Radiotherapy (SBRT) in the Treatment of Locally Advanced Pancreatic Cancer. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=NCT01926197 (2013).
National Institutes of Health. Randomized Phase 2 Study of mFOLFIRINOX With Or Without Stereotactic Body Radiotherapy in Patients With Locally Advanced Pancreatic Adenocarcinoma. NIH https://cris.nih.go.kr/cris/search/detailSearch.do/18961 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04998552 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03704662 (2023).
Tuli, R., David, J., Lobaugh, S., Zhang, Z. & O’Reilly, E. M. Duration of therapy for locally pancreatic advanced cancer: does it matter? Cancer Med. 9, 4572–4580 (2020).
Lee, M. et al. Impact of conversion surgery on survival in locally advanced pancreatic cancer patients treated with FOLFIRINOX chemotherapy. J. Hepatobiliary Pancreat. Sci. 30, 111–121 (2023).
Michelakos, T. et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann. Surg. 269, 733–740 (2019).
Alva-Ruiz, R. et al. Neoadjuvant chemotherapy switch in borderline resectable/locally advanced pancreatic cancer. Ann. Surg. Oncol. 29, 1579–1591 (2022).
Vreeland, T. J. et al. Benefit of gemcitabine/nab-paclitaxel rescue of patients with borderline resectable or locally advanced pancreatic adenocarcinoma after early failure of FOLFIRINOX. Pancreas 48, 837–843 (2019).
Maggino, L. et al. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. 154, 932–942 (2019).
Brown, Z. J. et al. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: meta-analysis. Br. J. Surg. 110, 34–42 (2022).
Kunzmann, V. et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 6, 128–138 (2021).
Williet, N. et al. Gemcitabine/nab-paclitaxel versus FOLFIRINOX in locally advanced pancreatic cancer: a european multicenter study. Cancers 13, 2797 (2021).
Yoo, C. et al. FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 12, 1758835920953294 (2020).
Hall, W. A. et al. Value of neoadjuvant radiation therapy in the management of pancreatic adenocarcinoma. J. Clin. Oncol. 39, 3773–3777 (2021).
Versteijne, E. et al. Neoadjuvant treatment for resectable and borderline resectable pancreatic cancer: chemotherapy or chemoradiotherapy? Front. Oncol. 11, 744161 (2022).
Palta, M. et al. Radiation therapy for pancreatic cancer: executive summary of an ASTRO clinical practice guideline. Pract. Radiat. Oncol. 9, 322–332 (2019).
Prasad, S. et al. Intensity modulated radiation therapy reduces gastrointestinal toxicity in locally advanced pancreas cancer. Pract. Radiat. Oncol. 6, 78–85 (2016).
Tchelebi, L. T. et al. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): an international systematic rview and meta-analysis. Cancer 126, 2120–2131 (2020).
Hill, C. S. et al. neoadjuvant stereotactic body radiotherapy after upfront chemotherapy improves pathologic outcomes compared with chemotherapy alone for patients with borderline resectable or locally advanced pancreatic adenocarcinoma without increasing perioperative toxicity. Ann. Surg. Oncol. 29, 2456–2468 (2022).
Ma, S. J. et al. Association of survival with stereotactic body radiation therapy following induction chemotherapy for unresected locally advanced pancreatic cancer. J. Radiother. Pract. 21, 403–410 (2022).
Amini, A. et al. Patterns of care for locally advanced pancreatic adenocarcinoma using the national cancer database. Pancreas 46, 904–912 (2017).
Krishnan, S. et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 94, 755–765 (2016).
Ng, S. P. & Koay, E. J. Current and emerging radiotherapy strategies for pancreatic adenocarcinoma: stereotactic, intensity modulated and particle radiotherapy. Ann. Pancreat. Cancer 1, 22 (2018).
Chapman, B. C. et al. Perioperative outcomes and survival following neoadjuvant stereotactic body radiation therapy (SBRT) versus intensity-modulated radiation therapy (IMRT) in pancreatic adenocarcinoma. J. Surg. Oncol. 117, 1073–1083 (2018).
Abi Jaoude, J. et al. Stereotactic versus conventional radiation therapy for patients with pancreatic cancer in the modern era. Adv. Radiat. Oncol. 6, 100763 (2021).
Hammel, P. et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 315, 1844–1853 (2016).
Katz, M. H. G. et al. Efficacy of preoperative mFOLFIRINOX vs mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: the A021501 phase 2 randomized clinical trial. JAMA Oncol. 8, 1263–1270 (2022).
Katz, M. H. G., Herman, J. M. & O’Reilly, E. M. Neoadjuvant mFOLFIRINOX vs mFOLFIRINOX plus radiotherapy in patients with borderline resectable pancreatic cancer-the A021501 trial-reply. JAMA Oncol. 9, 277–278 (2023).
Chopra, A. et al. Outcomes of neoadjuvant chemotherapy versus chemoradiation in localized pancreatic cancer: a case-control matched analysis. Ann. Surg. Oncol. 28, 3779–3788 (2021).
Vidri, R. J., Vogt, A. O., Macgillivray, D. C., Bristol, I. J. & Fitzgerald, T. L. Better defining the role of total neoadjuvant radiation: changing paradigms in locally advanced pancreatic cancer. Ann. Surg. Oncol. 26, 3701–3708 (2019).
Nagakawa, Y. et al. Clinical impact of neoadjuvant chemotherapy and chemoradiotherapy in borderline resectable pancreatic cancer: analysis of 884 patients at facilities specializing in pancreatic surgery. Ann. Surg. Oncol. 26, 1629–1636 (2019).
Janssen, Q. P. et al. Neoadjuvant radiotherapy after (m)FOLFIRINOX for borderline resectable pancreatic adenocarcinoma: a TAPS consortium study. J. Natl Compr. Canc. Netw. 20, 783–791.e1 (2022).
Pietrasz, D. et al. How does chemoradiotherapy following induction FOLFIRINOX improve the results in resected borderline or locally advanced pancreatic adenocarcinoma? An AGEO-FRENCH multicentric cohort. Ann. Surg. Oncol. 26, 109–117 (2019).
Auclin, E. et al. Role of FOLFIRINOX and chemoradiotherapy in locally advanced and borderline resectable pancreatic adenocarcinoma: update of the AGEO cohort. Br. J. Cancer 124, 1941–1948 (2021).
Ryckman, J. M. et al. The timing and design of stereotactic radiotherapy approaches as a part of neoadjuvant therapy in pancreatic cancer: is it time for change? Clin. Transl. Radiat. Oncol. 28, 124–128 (2021).
Torgeson, A. et al. Multiagent induction chemotherapy followed by chemoradiation is associated with improved survival in locally advanced pancreatic cancer. Cancer 123, 3816–3824 (2017).
De Simoni, O. et al. Could total neoadjuvant therapy followed by surgical resection be the new standard of care in pancratic cancer? A systematic review and meta-analysis. J. Clin. Med. 11, 812 (2022).
Faisal, F. et al. Longer course of induction chemotherapy followed by chemoradiation favors better survival outcomes for patients with locally advanced pancreatic cancer. Am. J. Clin. Oncol. 39, 18–26 (2016).
van der Geest, L. G. et al. Nationwide outcomes in patients undergoing surgical exploration without resection for pancreatic cancer. Br. J. Surg. 104, 1568–1577 (2017).
Chawla, A. et al. Prospective phase II trials validate the effect of neoadjuvant chemotherapy on pattern of recurrence in pancreatic adenocarcinoma. Ann. Surg. 276, e502–e509 (2022).
Dholakia, A. S. et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiograpic appearance of tumor-vessel relationship. J. Radiat. Oncol. 2, 413–424 (2013).
Ferrone, C. R. et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 261, 12–17 (2015).
Park, S. et al. CT in the prediction of margin-negative resection in pancreatic cancer following neoadjuvant treatment: a systematic review and meta-analysis. Eur. Radiol. 31, 3383–3393 (2021).
Yang, H. K. et al. Systematic review and meta‐analysis of diagnostic performance of CT imaging for assessing resectability of pancreatic ductal adenocarcinoma after neoadjuvant therapy: importance of CT criteria. Abdom. Radiol. 46, 5201–5217 (2021).
Soloff, E. et al. Imaging assessment of pancreatic cancer resectability after neoadjuvant therapy: ajr expert panel narrative review. AJR Am. J. Roentgenol. 218, 570–581 (2022).
Oba, A. et al. New criteria of resectability for pancreatic cancer: a position paper by the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS). J. Hepatobiliary Pancreat. Sci. 29, 725–731 (2022).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Perri, G. et al. Response and survival associated with first-line FOLFIRINOX vs gemcitabine and nab-paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. JAMA Surg. 155, 832–839 (2020).
Ahmed, S. A. et al. Preoperative CT staging of borderline pancreatic cancer patients after neoadjuvant treatment: accuracy in the prediction of vascular invasion and resectability. Abdom. Radiol. 46, 280–289 (2021).
Ahmed, S. A., Atta, H. & Hassan, R. A. The utility of multi-detector computed tomography criteria after neoadjuvant therapy in borderline resectable pancreatic cancer: prospective, bi-institutional study. Eur. J. Radiol. 139, 109685 (2021).
Noda, Y. et al. Arterial involvement and resectability scoring system to predict R0 resection in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation therapy. Eur. Radiol. 32, 2470–2480 (2022).
Habib, J. R. et al. Periadventitial dissection of the superior mesenteric artery for locally advanced pancreatic cancer: surgical planning with the “halo sign” and “string sign”. Surgery 169, 1026–1031 (2021).
Stoop, T. F. et al. Added value of 3T MRI and the MRI-halo sign in assessing resectability of locally advanced pancreatic cancer following induction chemotherapy (IMAGE-MRI): prospective pilot study. Langenbecks Arch. Surg. 407, 3487–3499 (2022).
Giannone, F. et al. Resectability of pancreatic cancer is in the eye of the observer — a multicenter, blinded, prospective assessment of interobserver agreement on NCCN resectability status criteria. Ann. Surg. Open 2, e087 (2021).
Kim, H. Y. et al. Tumor resectability and response on CT following neoadjuvant therapy for pancreatic cancer: inter-observer agreement study. Eur. Radiol. 32, 3799–3807 (2022).
Heckler, M. & Hackert, T. Surgery for locally advanced pancreatic ductal adenocarcinoma - is it only about the vessels? J. Gastrointest. Oncol. 12, 2503–2511 (2021).
Committee of the Korean Clinical Practice Guideline for Pancreatic Cancer and National Cancer Center, Korea. Korean Clinical Practice Guideline for Pancreatic Cancer 2021: a summary of evidence-based, multidisciplinary diagnostic and therapeutic approaches. Pancreatology 21, 1326–1341 (2021).
Ballehaninna, U. K. & Chamberlain, R. S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J. Gastrointest. Oncol. 3, 105–119 (2012).
Bergquist, J. R. et al. Carbohydrate antigen 19-9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a national cancer database study. J. Am. Coll. Surg. 223, 52–65 (2016).
Kinny-Köster, B., Habib, J., Wolfgang, C. L., He, J. & Javed, A. A. Favorable tumor biology in locally advanced pancreatic cancer — beyond CA19-9. J. Gastrointest. Oncol. 12, 2484–2494 (2021).
Aldakkak, M. et al. Pre-treatment carbohydrate antigen 19-9 does not predict the response to neoadjuvant therapy in patients with localized pancreatic cancer. HPB 17, 942–952 (2015).
Combs, S. E. et al. Prognostic impact of CA 19-9 on outcome after neoadjuvant chemoradiation in patients with locally advanced pancreatic cancer. Ann. Surg. Oncol. 21, 2801–2807 (2014).
Tzeng, C. W. et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB 16, 430–438 (2014).
Tsai, S. et al. Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann. Surg. 271, 740–747 (2020).
Newhook, T. E. et al. Prognosis associated with CA19-9 response dynamics and normalization during neoadjuvant therapy in resected pancreatic adenocarcinoma. Ann. Surg. 277, 484–490 (2023).
Maggino, L. et al. CA19.9 response and tumor size predict recurrence following post-neoadjuvant pancreatectomy in initially resectable and borderline resectable pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 30, 207–219 (2023).
Hartlapp, I. et al. Prognostic and predictive value of CA19-9 in locally advanced pancreatic cancer treated with multi-agent induction chemotherapy: results from a prospective, multicenter phase II trial (NEOLAP-AIO-PAK-0113). ESMO Open 7, 100552 (2022).
Heger, U. et al. Induction chemotherapy in pancreatic cancer: CA 19-9 may predict resectability and survival. HPB 22, 224–232 (2020).
Ye, C. et al. The prognostic value of CA19-9 response after neoadjuvant therapy in patients with pancreatic cancer: a systematic review and pooled analysis. Cancer Chemother. Pharmacol. 86, 731–740 (2020).
Lee, W. et al. Reduced and normalized carbohydrate antigen 19-9 concentrations after neoadjuvant chemotherapy have comparable prognostic performance in patients with borderline resectable and locally advanced pancreatic cancer. J. Clin. Med. 9, 1477 (2020).
van Veldhuisen, E. et al. Added value of CA19-9 response in predicting resectability of locally advanced pancreatic cancer following induction chemotherapy. HPB 20, 605–611 (2018).
Rose, J. B. et al. Sustained carbohydrate antigen 19-9 response to neoadjuvant chemotherapy in borderline resectable pancreatic cancer predicts progression and survival. Oncologist 25, 859–866 (2020).
Reni, M. et al. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann. Oncol. 28, 2786–2792 (2017).
Diab, H. M. H., Smith, H. G., Jensen, K. K. & Jørgensen, L. N. The current role of blood-based biomarkers in surgical decision-making in patients with localised pancreatic cancer: a systematic review. Eur. J. Cancer 154, 73–81 (2021).
Yang, Y. et al. Guidelines for the diagnosis and treatment of pancreatic cancer in China (2021). J. Pancreatol. 4, 49–66 (2021).
Luo, G. et al. Potential biomarkers in lewis negative patients with pancreatic cancer. Ann. Surg. 265, 800–805 (2017).
Omiya, K. et al. Serum DUPAN-2 could be an alternative biological marker for CA19-9 non-secretors with pancreatic cancer. Ann. Surg. https://doi.org/10.1097/SLA.0000000000005395 (2022).
Meng, Q. et al. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: a systematic review and meta-analysis. OncoTargets Ther. 10, 4591–4598 (2017).
Kato, H. et al. Role of serum carcinoma embryonic antigen (CEA) level in localized pancreatic adenocarcinoma: CEA level before operation is a significant prognostic indicator in patients with locally advanced pancreatic cancer treated with neoadjuvant therapy followed by surgical resection: a retrospective analysis. Ann. Surg. 275, e698–e707 (2022).
Wang, Z. J. et al. Therapeutic response assessment in pancreatic ductal adenocarcinoma: society of abdominal radiology review paper on the role of morphological and functional imaging techniques. Abdom. Radiol. 45, 4273–4289 (2020).
Marchegiani, G. et al. Surgery after FOLFIRINOX treatment for locally advanced and borderline resectable pancreatic cancer: increase in tumour attenuation on CT correlates with R0 resection. Eur. Radiol. 28, 4265–4273 (2018).
Kim, S. S., Lee, S., Lee, H. S., Bang, S. & Park, M. S. Prognostic factors in patients with locally advanced or borderline resectable pancreatic ductal adenocarcinoma: chemotherapy vs. chemoradiotherapy. Abdom. Radiol. 46, 655–666 (2021).
Okusaka, T. et al. Clinical practice guidelines for pancreatic cancer 2019 from the japan pancreas society: a synopsis. Pancreas 49, 326–335 (2020).
O’Reilly, D. et al. Diagnosis and management of pancreatic cancer in adults: a summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 18, 962–970 (2018).
Ghidini, M. et al. The role of positron emission tomography/computed tomography (PET/CT) for staging and disease response assessment in localized and locally advanced pancreatic cancer. Cancers 13, 4155 (2021).
Evangelista, L. et al. The role of FDG PET/CT or PET/MRI in assessing response to neoadjuvant therapy for patients with borderline or resectable pancreatic cancer: a systematic literature review. Ann. Nucl. Med. 35, 767–776 (2021).
Abdelrahman, A. M. et al. FDG-PET predicts neoadjuvant therapy response and survival in borderline resectable/locally advanced pancreatic adenocarcinoma. J. Natl Compr. Canc. Netw. 20, 1023–1032 (2022).
Akita, H. et al. FDG-PET predicts treatment efficacy and surgical outcome of pre-operative chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Eur. J. Surg. Oncol. 43, 1061–1067 (2017).
Akita, H. et al. Difference between carbohydrate antigen 19-9 and fluorine-18 fluorodeoxyglucose positron emission tomography in evaluating the treatment efficacy of neoadjuvant treatment in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma: results of a dual-center study. Ann. Gastroenterol. Surg. 5, 381–389 (2020).
Lee, W. et al. Metabolic activity by FDG-PET/CT after neoadjuvant chemotherapy in borderline resectable and locally advanced pancreatic cancer and association with survival. Br. J. Surg. 109, 61–70 (2021).
Barnes, C. A. et al. Value of pretreatment 18F-fluorodeoxyglucose positron emission tomography in patients with localized pancreatic cancer treated with neoadjuvant therapy. Front. Oncol. 10, 500 (2020).
Chang, J. S. et al. Clinical usefulness of 18F-fluorodeoxyglucose-positron emission tomography in patients with locally advanced pancreatic cancer planned to undergo concurrent chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 90, 126–133 (2014).
Padhani, A. R. et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11, 102–125 (2009).
Thoeny, H. C. & Ross, B. D. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J. Magn. Reson. Imaging 32, 2–16 (2010).
van Roessel, S. et al. Scoring of tumour response after neoadjuvant therapy in resected pancreatic cancer: systematic review. Br. J. Surg. 108, 119–127 (2021).
Murata, Y. et al. Clinical significance and predictors of complete or near-complete histological response to preoperative chemoradiotherapy in patients with localized pancreatic ductal adenocarcinoma. Pancreatology 21, 1482–1490 (2021).
Wittmann, D. et al. Impact of neoadjuvant chemoradiation on pathologic response in patients with localized pancreatic cancer. Front. Oncol. 10, 460 (2020).
Truty, M. J. et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann. Surg. 273, 341–349 (2021).
Sell, N. M. et al. Evaluation of pathologic response on overall survival after neoadjuvant therapy in pancreatic ductal adenocarcinoma. Pancreas 49, 897–903 (2020).
Maeda, S. et al. Pathological treatment response has different prognostic implications for pancreatic cancer patients treated with neoadjuvant chemotherapy or chemoradiotherapy. Surgery 171, 1379–1387 (2022).
Antolino, L. et al. Is complete pathological response in pancreatic cancer overestimated? A systematic review of prospective studies. J. Gastrointest. Surg. 24, 2336–2348 (2020).
Cloyd, J. M. et al. Pathological complete response following neoadjuvant therapy for pancreatic ductal adenocarcinoma: defining the incidence, predictors, and outcomes. HPB 22, 1569–1576 (2020).
Barrak, D. et al. Total neoadjuvant therapy for pancreatic adenocarcinoma increases probability for a complete pathologic response. Eur. J. Surg. Oncol. 48, 1356–1361 (2022).
He, J. et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer. Ann. Surg. 268, 1–8 (2018).
Blair, A. B. et al. Recurrence in patients achieving pathological complete response after neoadjuvant treatment for advanced pancreatic cancer. Ann. Surg. 274, 162–169 (2021).
Janssen, B. V. et al. Histopathological tumour response scoring in resected pancreatic cancer following neoadjuvant therapy: international interobserver study (ISGPP-1). Br. J. Surg. 110, 67–75 (2022).
Janssen, B. V. et al. Amsterdam international consensus meeting: tumor response scoring in the pathology assessment of resected pancreatic cancer after neoadjuvant therapy. Mod. Pathol. 34, 4–12 (2021).
Janssen, B. V. et al. Artificial intelligence-based segmentation of residual tumor in histopathology of pancreatic cancer after neoadjuvant treatment. Cancers 13, 5089 (2021).
Sandini, M. et al. Association between changes in body composition and neoadjuvant tratment for pancreatic cancer. JAMA Surg. 153, 809–815 (2018).
Griffin, O. M. et al. Characterising the impact of body composition change during neoadjuvant chemotherapy for pancreatic cancer. Pancreatology 19, 850–857 (2019).
Weniger, M. et al. Respect — a multicenter retrospective study on preoperative chemotherapy in locally advanced and borderline resectable pancreatic cancer. Pancreatology 20, 1131–1138 (2020).
Brada, L. J. H. et al. Survival benefit associated with resection of locally advanced pancreatic cancer after upfront FOLFIRINOX versus FOLFIRINOX only: multicenter propensity score-matched analysis. Ann. Surg. 274, 729–735 (2021).
Macedo, F. I. et al. Survival outcomes associated with clinical and pathological response following neoadjuvant FOLFIRINOX or gemcitabine/nab-paclitaxel chemotherapy in resected pancreatic cancer. Ann. Surg. 270, 400–413 (2019).
Rangelova, E. et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann. Surg. 273, 579–586 (2021).
Janssen, Q. P. et al. FOLFIRINOX as initial treatment for localized pancreatic adenocarcinoma: a retrospective analysis by the Trans-Atlantic Pancreatic Surgery Consortium. J. Natl Cancer Inst. 114, 695–703 (2022).
De Rosa, A., Cameron, I. C. & Gomez, D. Indications for staging laparoscopy in pancreatic cancer. HPB 18, 13–20 (2016).
Takadate, T. et al. Staging laparoscopy is mandatory for the treatment of pancreatic cancer to avoid missing radiologically negative metastases. Surg. Today 51, 686–694 (2021).
Suker, M. et al. Yield of staging laparoscopy before treatment of locally advanced pancreatic cancer to detect occult metastases. Eur. J. Surg. Oncol. 45, 1906–1911 (2019).
Fong, Z. V. et al. Reappraisal of staging laparoscopy for patients with pancreatic adenocarcinoma: a contemporary analysis of 1001 patients. Ann. Surg. Oncol. 24, 3203–3211 (2017).
Gemenetzis, G. et al. Incidence and risk factors for abdominal occult metastatic disease in patients with pancreatic adenocarcinoma. J. Surg. Oncol. 118, 1277–1284 (2018).
Sakaguchi, T. et al. A simple risk score for detecting radiological occult metastasis in patients with resectable or borderline resectable pancreatic ductal adenocarcinoma. J. Hepatobiliary Pancreat. Sci. 29, 262–270 (2022).
Oba, A. et al. Staging laparoscopy for pancreatic cancer using intraoperative ultrasonography and fluoresence imaging: the SLING trial. Br. J. Surg. 108, 115–118 (2021).
Peng, J. S. et al. Diagnostic laparoscopy prior to neoadjuvant therapy in pancreatic cancer is high yield: an analysis of outcomes and costs. J. Gastrointest. Surg. 21, 1420–1427 (2017).
Paracha, M. et al. Opportunity lost? diagnostic laparoscopy in patients with pancreatic cancer in the national surgical quality improvement program database. World J. Surg. 43, 937–943 (2019).
van Veldhuisen, E. et al. Added value of intra-operative ultrasound to determine the resectability of locally advanced pancreatic cancer following FOLFIRINOX chemotherapy (IMAGE): a prospective multicenter study. HPB 21, 1385–1392 (2019).
Diener, M. K. et al. Periarterial divestment in pancreatic cancer surgery. Surgery 169, 1019–1025 (2021).
Walma, M. S. et al. Treatment strategies and clinical outcomes in consecutive patients with locally advanced pancreatic cancer: a multicenter prospective cohort. Eur. J. Surg. Oncol. 20, 699–707 (2021).
Hackert, T. et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann. Surg. 264, 457–463 (2016).
Raptis, D. A. et al. Defining benchmark outcomes for pancreaticoduodenectomy with concomitant portomesenteric venous resection. Ann. Surg. 272, 731–737 (2020).
Bockhorn, M. et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 155, 977–988 (2014).
Machairas, N. et al. The impact of neoadjuvant treatment on survival in patients undergoing pancreatoduodenectomy with concomitant portomesenteric venous resection: an international multicenter analysis. Ann. Surg. 274, 721–728 (2021).
Groen, J. V. et al. Venous wedge and segment resection during pancreatoduodenectomy for pancreatic cancer: impact on short- and long-term outcomes in a nationwide cohort analysis. Br. J. Surg. 109, 96–104 (2021).
Hackert, T. et al. Portal vein resection in pancreatic cancer surgery: risk of thrombosis and radicality determine survival. Ann. Surg. https://doi.org/10.1097/SLA.0000000000005444 (2022).
Kinny-Köster, B. et al. Mesoportal bypass, interposition graft, and mesocaval shunt: surgical strategies to overcome superior mesenteric vein involvement in pancreatic cancer. Surgery 168, 1048–1055 (2020).
Oba, A. et al. Extent of venous resection during pancreatectomy - finding the balance of technical possiblity and feasibility. J. Gastrointest. Oncol. 12, 2495–2502 (2021).
Schneider, M., Hackert, T., Strobel, O. & Büchler, M. W. Technical advances in surgery for pancreatic cancer. Br. J. Surg. 108, 777–785 (2021).
Inoue, Y. et al. Optimal extent of superior mesenteric artery dissection during pancreaticoduodenectomy for pancreatic cancer: balancing surgical and oncological safety. J. Gastrointest. Surg. 23, 1373–1383 (2019).
Loos, M. et al. Arterial resection in pancreatic cancer surgery: effective after a learning curve. Ann. Surg. 275, 759–768 (2022).
Del Chiaro, M. & Schulick, R. D. Commentary on: divestment or skeletonization of the SMA or the hepatic artery for locally advanced pancreatic ductal cancer after neoadjuvant therapy. Surgery 169, 1039–1040 (2020).
Stoop, T. F. et al. Pancreatectomy with arterial resection for periampullary cancer: outcomes after planned or unplanned events in a nationwide, multicentre cohort. Br. J. Surg. 110, 638–642 (2023).
Ferrone, C. R. Divestment/skeletonization of the arteries in patients with advanced pancreatic ductal cancer. Surgery 169, 1037–1038 (2021).
Mollberg, N. et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann. Surg. 254, 882–893 (2011).
Rebelo, A. et al. Systematic review and meta-analysis of contemporary pancreas surgery with arterial resection. Langenbecks Arch. Surg. 405, 903–919 (2020).
Tee, M. C. et al. Indications and perioperative outcomes for pancreatectomy with arterial resection. J. Am. Coll. Surg. 227, 255–269 (2018).
Sonohara, F. et al. Novel implications of combined arterial resection for locally advanced pancreatic cancer in the era of newer chemo-regimens. Eur. J. Surg. Oncol. 45, 1895–1900 (2019).
Del Chiaro, M. et al. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB 21, 219–225 (2019).
Bachellier, P., Addeo, P., Faitot, F., Nappo, G. & Dufour, P. Pancreatectomy with arterial resection for pancreatic adenocarcinoma: how can it be done safely and with which outcomes?: a single institution’s experience with 118 patients. Ann. Surg. 271, 932–940 (2020).
Schmocker, R. K. et al. An aggressive approach to locally confined pancreatic cancer: defining surgical and oncological outcomes unique to pancreatectomy with celiac axis resection (DP-CAR). Ann. Surg. Oncol. 28, 3125–3134 (2021).
Boggi, U. et al. Pancreatectomy with resection and reconstruction of the superior mesenteric artery. Br. J. Surg. 110, 901–904 (2023).
Alva-Ruiz, R. et al. Patency rates of hepatic arterial resection and revascularization in locally advanced pancreatic cancer. HPB 24, 1957–1966 (2022).
Kinny-Koster, B. et al. Conduits in vascular pancreatic surgery: analysis of clinical outcomes, operative techniques and graft performance. Ann. Surg. 278, e94–e104 (2023).
Wiltberger, G. et al. Perioperative and long-term outcome of en-bloc arterial resection in pancreatic surgery. HPB 24, 1119–1128 (2022).
Klompmaker, S. et al. Outcomes after distal pancreatectomy with celiac axis resection for pancreatic cancer: a pan-european retrospective cohort study. Ann. Surg. Oncol. 25, 1440–1447 (2018).
Truty, M. J. et al. En bloc celiac axis resection for pancreatic cancer: classification of anatomical variants based on tumor extent. J. Am. Coll. Surg. 231, 8–29 (2020).
Addeo, P., Guerra, M. & Bachellier, P. Distal pancreatectomy with en bloc celiac axis resection (DP-CAR) and arterial reconstruction: techniques and outcomes. J. Surg. Oncol. 123, 1592–1598 (2021).
Napoli, N. et al. Factors predicting survival in patients with locally advanced pancreatic cancer undergoing pancreatectomy with arterial resection. Updates Surg. 73, 233–249 (2021).
Christians, K. K. et al. Arterial resection at the time of pancreatectomy for cancer. Surgery 155, 919–926 (2014).
Del Chiaro, M., Rangelova, E., Segersvard, R. & Arnelo, U. Are there still indications for total pancreatectomy? Updates Surg. 68, 257–263 (2016).
Stoop, T. F. et al. Total pancreatectomy as an alternative to high-risk pancreatojejunostomy after pancreatoduodenectomy: a propensity score analysis on surgical outcome and quality of life. HPB 24, 1261–1270 (2022).
Marchegiani, G. et al. High-risk pancreatic anastomosis vs. total pancreatectomy after pancreatoduodenectomy. Ann. Surg. 276, e905–e913 (2022).
Garnier, J. et al. Pancreatectomy with vascular resection after neoadjuvant FOLFIRINOX: who survives more than a year after surgery? Ann. Surg. Oncol. 28, 4625–4634 (2021).
de Geus, S. W. L. et al. Neoadjuvant therapy affects margins and margins affect all: perioperative and survival outcomes in resected pancreatic adenocarcinoma. HPB 20, 573–581 (2018).
Schmocker, R. K. et al. Impact of margin status on survival in patients with pancreatic ductal adenocarcinoma receiving neoadjuvant chemotherapy. J. Am. Coll. Surg. 232, 405–413 (2021).
Klaiber, U. et al. Prognostic factors of survival after neoadjuvant treatment and resection for initially unresectable pancreatic cancer. Ann. Surg. 273, 154–162 (2021).
Cai, B. et al. Sub-adventitial divestment technique for resecting artery-involved pancreatic cancer: a retrospective cohort study. Langenbecks Arch. Surg. 406, 691–701 (2021).
Mirkin, K. A., Hollenbeak, C. S., Gusani, N. J. & Wong, J. Trends in utilization of neoadjuvant therapy and short-term outcomes in resected pancreatic cancer. Am. J. Surg. 214, 80–88 (2017).
Kamarajah, S. K., Naffouje, S. A., Salti, G. I. & Dahdaleh, F. S. Neoadjuvant chemotherapy for pancreatic ductal adenocarcinoma is associated with lower post-pancreatctomy readmission rates: a population-based cohort study. Ann. Surg. Oncol. 28, 1896–1905 (2021).
Oba, A. et al. Comparing neoadjuvant chemotherapy with or without radiation therapy for pancreatic ductal adenocarcinoma: National Cancer Database cohort analysis. Br. J. Surg. 109, 450–454 (2022).
van Dongen, J. C. et al. The effect of preoperative chemotherapy and chemoradiotherapy on pancreatic fistula and other surgical complications after pancreatic resection: a systematic review and meta-analysis of comparative studies. HPB 23, 1321–1331 (2021).
Marchegiani, G. et al. Neoadjuvant therapy versus upfront resection for pancreatic cancer: the actual spectum and clinical burden of postoperative complications. Ann. Surg. Oncol. 25, 626–637 (2018).
Del Chiaro, M. & Schulick, R. D. Use of total pancreatectomy and preoperative radiotherapy in patients undergoing pancreatectomy with arterial resection. J. Am. Coll. Surg. 228, 131 (2019).
Blair, A. B. et al. Postoperative complications after resection of borderline resectable and locally advanced pancreatic cancer: the impact of neoadjuvant chemotherapy with conventional radiation or stereotactic body radiation therapy. Surgery 163, 1090–1096 (2018).
Iacobuzio-Donahue, C. A. et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J. Clin. Oncol. 27, 1806–1813 (2009).
White, R. R., Murphy, J. D. & Martin, R. C. G. The landmark series: locally advanced pancreatic cancer and ablative therapy options. Ann. Surg. Oncol. 28, 4173–4180 (2021).
Heger, U. & Hackert, T. Can local ablative techniques replace surgery for locally advanced pancreatic cancer? J. Gastrointest. Oncol. 12, 2536–2546 (2021).
Jolissaint, J. S. et al. Local control and survival after induction chemotherapy and ablative radiation versus resection for pancreatic ductal adenocarcinoma with vascular involvement. Ann. Surg. 274, 894–901 (2021).
Chuong, M. D. et al. Induction chemotherapy and ablative stereotactic magnetic resonance image-guided adaptive radiation therapy for inoperable pancreas cancer. Front. Oncol. 12, 888462 (2022).
Frigerio, I. et al. Open radiofrequency ablation as upfront treatment for locally advanced pancreatic cancre: requiem from a randomized controlled trial. Pancreatology 21, 1342–1348 (2021).
Lin, M. et al. Irreversible electroporation plus allogenic Vγ9Vδ2 T cells enhances antitumor effect for locally advanced pancreatic cancer. Signal. Transduct. Target. Ther. 5, 215 (2020).
Walma, M. S. et al. Radiofrequency ablation and chemotherapy versus chemotherapy alone for locally advanced pancreatic cancer (PELICAN): study protocol for a randomized controlled trial. Trials 22, 313 (2021).
Academic Medical Center. Assessing Optimal Treatment Settings for the Ablation of Locally Advanced Pancreatic Cancer With Ct-Guided Percutaneous Irreversibl Electroporation (Antilope): A Randomized Feasibility Study. Academic Medical Centerhttps://www.trialregister.nl/trial/7015 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02791503 (2022).
World Health Organization. A Comparison of Combined Eus-Guided Radiofrequency Ablation Using EUSRA RF Electrode and Chemotherapy vs. Chemotherapy Alone in Locally Advanced Pancreatic Cancer: A Phase II/III Randomized Controlled Trial. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20201223001 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03899636?term=IRE&recrs=abdefgh&type=Intr&cond=Pancreatic+Cancer&age=1&draw=2&rank=10 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02343835?term=IRE&recrs=abdefgh&type=Intr&cond=Pancreatic+Cancer&age=1&draw=1&rank=7 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04699539?term=SBRT&recrs=abdefgh&type=Intr&cond=Pancreatic+Cancer&age=1&draw=3 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04603586?term=SBRT&recrs=abdefgh&type=Intr&cond=Pancreatic+Cancer&age=1&draw=3&rank=41 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01921751?term=IMRT&recrs=abdefgh&type=Intr&cond=Pancreatic+Cancer&age=1&draw=2&rank=12 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03673137 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02336672 (2023).
World Health Organization. Treatment of Unresectable Locally Advanced Pancreas Cancer With Percutaneous Irreversible Electroporation Following Initial Systemic Chemotherapy (LAP-PIE): A Randomised Controlled Feasibility Trial. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN14986389 (2021).
World Health Organization. A Prospective, Randomized, Phase 3 Trial of Carbon Ion Radiation Therapy Versus Standard Care for Locally Advanced Pancreatic Cancer. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=NCT04592861 (2020).
Izzo, F. et al. A multicenter randomized controlled prospective study to assess efficacy of laparoscopic electrochemotherapy in the treatment of locally advanced pancreatic cancer. J. Clin. Med. 10, 4011 (2021).
World Health Organization. Clinical Study of S-1 Chemotherapy Combined with TOMO Radiotherapy as the First-Line Therapy for Locally Advanced Pancreatic Cancer. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-IPR-16009949 (2016).
World Health Organization. The Efficacy and Safety of High Dose and Low Fractionated Radiation Therapy for Locally Advanced Pancreatic Cancer: A Prospective, Randomized, Controlled, Multicenter Clinical Trial. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-IIR-16008875 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05466799 (2023).
World Health Organization. Prospective Randomized Comparison of Endoscopic Ultrasound-Guided Radiofrequency Ablation (RFA) and mFOLFIRINOX Parallel Therapy And Chemotherapy Monotherapy In Patients With Pancreatic Cancer. WHO https://trialsearch.who.int/Trial2.aspx?TrialID=KCT0007349 (2022).
Doppenberg, D. et al. Stereotactic ablative radiotherapy or best supportive care in patients with localized pancreatic cancer not receiving chemotherapy and surgery (PANCOSAR): a nationwide multicenter randomized controlled trial according to a TwiCs design. BMC Cancer 22, 1363 (2022).
Molinari, M. et al. Patients’ treatment preferences for potentially resectable tumors of the head of the pancreas. HPB 22, 265–274 (2020).
Ziebland, S., Chapple, A. & Evans, J. Barriers to shared decisions in the most serious of cancers: a qualitative study of patients with pancreatic cancer treated in the UK. Health Expect. 18, 3302–3312 (2015).
Mihaljevic, A. L. et al. Not all Whipple procedures are equal: proposal for a classification of pancreatoduodenectomies. Surgery 169, 1456–1462 (2021).
Loos, M. et al. Categorization of different types of total pancreatectomy. JAMA Surg. 157, 120–128 (2022).
Scholten, L. et al. Systematic review of functional outcome and quality of life after total pancreatectomy. Br. J. Surg. 106, 1735–1746 (2019).
Kuroki, N. et al. Long-term outcome of patients with postoperative refractory diarrhea after tailored nerve plexus dissection around the major visceral arteries during pancreatoduodenectomy for pancreatic cancer. World J. Surg. 46, 1172–1182 (2022).
Scholten, L. et al. New-onset diabetes after pancreatoduodenectomy: a systematic review and meta-analysis. Surgery 164, 6–16 (2018).
Yu, J., Sun, R., Han, X. & Liu, Z. New-onset diabetes mellitus after distal pancreatectomy: a systematic review and meta-analysis. J. Laparoendosc. Adv. Surg. Tech. A 30, 1215–1222 (2020).
Moore, J. V. et al. Exocrine pancreatic insufficiency after pancreatectomy for malignancy: systematic review and optimal management recommendations. J. Gastrointest. Surg. 25, 2317–2327 (2021).
Griffioen, I. P. M. et al. The bigger picture of shared decision making: a service design perspective using the care path of locally advanced pancreatic cancer as a case. Cancer Med. 10, 5907–5916 (2021).
Mackay, T. M. et al. Patient satisfaction and quality of life before and after treatment of pancreatic and periampully cancer: a prospective multicenter study. J. Natl Compr. Canc. Netw. 18, 704–711 (2020).
Connor, A. A. & Gallinger, S. Pancreatic cancer evolution and heterogeneity: integrating omics and clinical data. Nat. Rev. Cancer 22, 131–142 (2022).
Hosein, A. N., Dougan, S. K., Aguirre, A. J. & Maitra, A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat. Cancer 3, 272–286 (2022).
Sivapalan, L., Kocher, H. M., Ross-Adams, H. & Chelala, C. The molecular landscape of pancreatic ductal adenocarcinoma. Pancreatology 22, 925–936 (2022).
Ecker, B. L. et al. Alterations in somatic driver genes are associated with response to neoadjuvant FOLFIRINOX in patients with localized pancreatic ductal adenocarcinoma. J. Am. Coll. Surg. 235, 342–349 (2022).
Suurmeijer, J. A. et al. Impact of classical and basal-like molecular subtypes on overall survival in resected pancreatic cancer in the SPACIOUS-2 multicentre study. Br. J. Surg. 109, 1150–1155 (2022).
Aung, K. L. et al. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin. Cancer Res. 24, 1344–1354 (2018).
Golan, T. et al. Increased rate of complete pathological response after neoadjuvant FOLFIRINOX for BRCA mutation carriers with borderline resectable pancreatic cancer. Ann. Surg. Oncol. 27, 3963–3970 (2020).
Tsai, S. et al. A phase II clinical trial of molecular profiled neoadjuvant therapy for localized pancreatic ductal adenocarcinoma. Ann. Surg. 268, 610–619 (2018).
Matsumoto, I. et al. FOLFIRINOX for locally advanced pancreatic cancer: results and prognostic factors of subset analysis from a nation-wide multicenter observational study in Japan. Pancreatology 19, 296–301 (2019).
Ueberroth, B. E., Jones, J. C. & Bekaii-Saab, T. S. Circulating tumor DNA (ctDNA) to evaluate minimal residual disease (MRD), treatment response, and posttreatment prognosis in pancreatic adenocarcinoma. Pancreatology 22, 741–748 (2022).
Janssen, B. V. et al. Imaging-based machine-learning models to predict clinical outcomes and identify biomarkers in pancreatic cancer: a scoping review. Ann. Surg. 275, 560–567 (2022).
van Eijck, C. W. F. et al. A multigene circulating biomarker to predict the lack of FOLFIRINOX response after a single cycle in patients with pancreatic ductal adenocarcinoma. Eur. J. Cancer 181, 119–134 (2022).
Farshadi, E. A. et al. Organoids derived from neoadjuvant FOLFIRINOX patients recapitulate therapy resistance in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 27, 6602–6612 (2021).
van der Sijde, F. et al. Circulating TP53 mutations are associated with early tumor progression and poor survival in pancreatic cancer patients treated with FOLFIRINOX. Ther. Adv. Med. Oncol. 13, 17588359211033704 (2021).
van der Sijde, F. et al. Serum cytokine levels are associated with tumor progression during FOLFIRINOX chemotherapy and overall survival in pancreatic cancer patients. Front. Immunol. 13, 898498 (2022).
van der Sijde, F. et al. Serum miR-373-3p and miR-194-5p are associated with early tumor progression during FOLFIRINOX treatment in pancreatic cancer patients: a prospective multicenter study. Int. J. Mol. Sci. 22, 10902 (2021).
Loehrer, P. J. Sr et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 29, 4105–4112 (2011).
Rich, T. A. et al. Weekly paclitaxel, gemcmitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer. Onco. Targets Ther. 5, 161–170 (2012).
Mukherjee, S. et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 14, 317–326 (2013).
Hurt, C. N. et al. Long-term results and recurrence patterns from SCALOP: a phase II randomised trial of gemcitabine- or capecitabine-based chemoradiation for locally advanced pancreatic cancer. Br. J. Cancer 116, 1264–1270 (2017).
Khan, K. et al. miR-21 expression and clinical outcome in locally advanced pancreatic cancer: exploratory analysis of the pancreatic cancer Erbitux, radiotherapy and UFT (PERU) trial. Oncotarget 7, 12672–12681 (2016).
Evans, T. R. J. et al. Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer. Ann. Oncol. 28, 354–361 (2017).
Picozzi, V. et al. Gemcitabine/nab-paclitaxel with pamrevlumab: a novel drug combination and trial design for the treatment of locally advanced pancreatic cancer. ESMO Open. 5, e000668 (2020).
Ioka, T. et al. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncoogy Group trial, JCOG1106. Jpn J. Clin. Oncol. 51, 235–243 (2021).
Liermann, J. et al. Cetuximab, gemcitabine and radiotherapy in locally advanced pancreatic cancer: long-term results of the randomized controlled phase II PARC trial. Clin. Transl. Radiat. Oncol. 34, 15–22 (2022).
Landry, J. et al. Randomized phase II study of gemcitabine plus radiotherpay versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J. Surg. Oncol. 101, 587–592 (2010).
Sahora, K. et al. A phase II trial of two durations of bevacizumab added to neoadjuvant gemcitabine for borderline and locally advanced pancreatic cancer. Anticancer. Res. 34, 2377–2384 (2014).
Hewitt, D. B. et al. A phase 3 randomized clinical trial of chemotherapy with or without algenpantucel-L (HyperAcute-Pancreas) immunotherapy in subjects with borderline resectable or locally advanced unresectable pancreatic cancer. Ann. Surg. 275, 45–53 (2022).
Acknowledgements
The authors thank Faridi S. van Etten-Jamaludin (Clinical Librarian, Amsterdam UMC, location University of Amsterdam) for her contribution to the construction of the literature search strategy.
Author information
Authors and Affiliations
Consortia
Contributions
M.G.B. and T.F.S. researched data for the article, made a substantial contribution to the discussion of content, wrote the article, and reviewed/edited the manuscript before submission. R.T.T. and L.W.F.S. researched data for the article, made a substantial contribution to the discussion of content, and reviewed/edited the manuscript before submission. The other authors reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.D.C. received an industry grant (Haemonetics, Inc.) to conduct a multicentre study to evaluate the prognostic implications of TEG in pancreatic cancer. M.D.C. is co-principal investigator of a Boston Scientific-sponsored international multicentre study on the use of intraoperative pancreatoscopy of patients with intraductal papillary mucinous neoplasms. T.F.S. and M.G.B. received two grants from the Dutch Cancer Society (KWF) and Deltaplan Alvleesklierkanker for the Dutch PREOPANC-4 trial on the multidisciplinary management of locally advanced pancreatic cancer (NCT05524090).
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Helmut Friess and Giuseppe Malleo for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
A systematic literature search on PubMed was performed (30 November 2022); see Supplementary Table 1 for the search strategy. Literature (n = 2,978 records) was screened by title and abstract (by T.F.S., L.W.F.S. and R.T.T.) and the preliminary articles included (n = 801) were subsequently screened by full-text (by L.W.F.S. and R.T.T.). Additional literature was identified via the references of included literature to further nuance certain topics. In addition, the WHO International Clinical Trials Registry Platform was searched (6 December 2022) for ongoing and completed randomized controlled trials. Trials about advanced pancreatic cancer were not included. On 6 February 2023, the status of included ongoing or completed trials was checked. Inclusion criteria concerned any type of original studies about preoperative chemotherapy or chemoradiotherapy for borderline resectable pancreatic cancer and/or locally advanced pancreatic cancer (LAPC; any definition), published in the period 2010(1) to 2022(11). Studies were included when they reported about (1) indications for preoperative chemotherapy or chemoradiotherapy or subsequent patient selection; and/or (2) (radical) resection rate and/or overall survival. Randomized controlled trials were included whereas primarily only observational studies with a sample size of ≥50 patients with borderline resectable pancreatic cancer and/or LAPC were selected. Single-centre studies with at least 200 patients with LAPC and multicentre studies with at least 100 patients were specifically included. Finally, smaller series were used to illustrate certain topics when larger series were not available.
Supplementary information
Glossary
- 5-Fluorouracil
-
(5-FU). A single-agent chemotherapy.
- ABC
-
(Anatomical – Biological – Conditional). Multi-domain parameters that are used to (re)stage patients with pancreatic cancer before and after preoperative therapy. This includes, among others, vascular tumour involvement (anatomical), tumour markers (biological) and patient fitness (condition).
- Adjuvant therapy
-
Adjuvant therapy for patients with pancreatic cancer often concerns systemic chemotherapy (with or without radiotherapy) that is given with a curative intention after surgery.
- Adverse events
-
Adverse events can be classified following the Common Terminology Criteria for Adverse Events, ranging from grade 1 (mild) and grade 2 (moderate) to grade 3 (severe or medically significant), grade 4 (life-threatening) or grade 5 (death).
- American Joint Committee on Cancer
-
(AJCC). The AJCC developed a staging system for pancreatic cancer, comprising the three-tier system T stage (tumour), N stage (lymph nodes) and M stage (distant metastases).
- Arterial divestment
-
A surgical technique whereby the (tumour) tissue is peeled off from an artery, without the need for an arterial resection.
- Borderline resectable pancreatic cancer
-
(BRPC). Resectability of a pancreatic tumour is often based on the presence and extent of vascular tumour involvement. Biological and conditional factors can also be part of resectability criteria. A borderline resectable pancreatic tumour means that the benefit of removing the tumour by surgery is uncertain or debatable.
- BRPC-A
-
Borderline resectable pancreatic cancer (BRPC) due to the presence of arterial involvement (that is, superior mesenteric artery, coeliac axis and/or hepatic artery).
- BRPC-PV
-
Borderline resectable pancreatic cancer (BRPC) due to the presence and extent of tumour involvement with the portomesenteric axis (that is, portal vein, confluence and superior mesenteric vein).
- Capecitabine
-
A single-agent chemotherapy.
- Carbohydrate antigen 19-9
-
(CA19-9). A serological tumour marker that is measurable in 80–85% of patients with pancreatic cancer. It is the most commonly used tumour marker in patients with pancreatic cancer.
- Carbohydrate antigen 125
-
(CA125). A serological tumour marker that might be of clinical value in patients with pancreatic cancer.
- Carcinoembryonic antigen
-
(CEA). A serological tumour marker that is elevated in 30–60% of patients with pancreatic cancer and could be of clinical value.
- Coeliac axis
-
The arterial branch that originates from the aorta and trifurcates into the common hepatic artery, left gastric artery and splenic artery in case of normal arterial anatomy.
- Complete pathological response
-
The absence of vital tumour cells in the resection specimen in response to therapy.
- Computed tomography
-
(CT). A type of cross-sectional imaging.
- Cross-sectional imaging
-
Advanced imaging modalities such as computed tomography and magnetic resonance imaging.
- Diffusion-weighted MRI
-
A specific modality of magnetic resonance imaging (MRI).
- Duke pancreatic monoclonal antigen type 2
-
(DUPAN2). A serological tumour marker that might be of clinical value in patients with pancreatic cancer.
- Eastern Cooperative Oncology Group (ECOG) performance status
-
A classification to indicate the condition of a patient, ranging from grade 0 (fully active) to grade 5 (deceased).
- External beam radiotherapy
-
(EBRT). A conventional radiation modality.
- Extrapancreatic disease
-
(Suspected) pancreatic cancer located outside the pancreas (that is, lymphadenopathy and/or distant metastases).
- FDG-PET
-
Fluorodeoxyglucose-positron emission tomography (PET) is combined with either computed tomography or magnetic resonance imaging.
- FOLFIRINOX
-
Multi-agent chemotherapy, comprising a combination of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin.
- Gemcitabine
-
A single-agent chemotherapy.
- Gemcitabine-capecitabine
-
A multi-agent chemotherapy, comprising gemcitabine and capecitabine.
- Gemcitabine-oxaliplatin
-
A multi-agent chemotherapy, comprising gemcitabine and oxaliplatin.
- Gemcitabine plus nab-paclitaxel
-
A multi-agent chemotherapy, comprising gemcitabine and albumin-bound paclitaxel.
- Gemcitabine-S1
-
A multi-agent chemotherapy, comprising gemcitabine and S1.
- Histopathological tumour response
-
The presence (and extent) of tumour response on preoperative therapy in the resected specimen can be assessed and graded.
- Hypofractionated image-guided radiotherapy
-
A type of radiation therapy that enables higher and more precise dosage whereby surrounding health tissue is spared.
- Induction therapy
-
Preoperative therapy for patients with locally advanced pancreatic cancer, using chemotherapy with or without radiation.
- Intensity-modulated radiotherapy
-
(IMRT). A type of radiation therapy that enables higher and more precise dosage whereby surrounding health tissue is spared.
- Irinotecan
-
A single-agent chemotherapeutic drug but often used as part of the multi-agent chemotherapy FOLFIRINOX.
- Localized pancreatic cancer
-
Pancreatic cancer without signs of metastatic disease.
- Locally advanced pancreatic cancer
-
(LAPC). Resectability of a pancreatic tumour is often based on the presence and extent of vascular tumour involvement. A locally advanced pancreatic tumour means that the tumour has sufficient contact with major peri-pancreatic vasculature that an upfront surgical resection is associated with significant risks.
- Magnetic resonance imaging
-
(MRI). A type of cross-sectional imaging.
- Maximum standard uptake value
-
(SUVmax). A measure of the highest metabolic activity on a fluorodeoxyglucose-positron emission tomography scan.
- Neoadjuvant therapy
-
Preoperative therapy for patients with (borderline) resectable pancreatic cancer, using chemotherapy with or without radiation.
- Pancreatic fistula
-
Leakage of anastomosis from the pancreas with stomach or jejunum, whereby pancreatic enzymes leak into the abdominal cavity.
- Pancreatoduodenectomy
-
Resection of the pancreatic head, duodenum, gallbladder and proximal jejunum, eventually combined with resection of the distal stomach.
- ‘Pick-the-winner’ design
-
A method that can be used for randomized controlled trials in which the best treatment of choice is chosen, also weighing alongside the efficacy factors such as toxicity, quality of life and health-care costs.
- Portomesenteric venous axis
-
The venous system that drains blood from the small bowel and colon to the liver. The main branches that are most relevant for the resectability from pancreatic cancer are the portal vein, confluence and superior mesenteric vein.
- Primary resectable pancreatic cancer
-
(PRPC). Resectability of a pancreatic tumour is often based on the presence and extent of vascular tumour involvement. A primary resectable pancreatic tumour means that it seems beneficial to remove the tumour by surgery.
- R0
-
R status indicates whether the resection margins are free from vital tumour cells: R0, margins are microscopically tumour-free.
- R1
-
R status indicates whether the resection margins are free from vital tumour cells: R1, margins are microscopically (closely) involved by vital tumour.
- R2
-
R status indicates whether the resection margins are free from vital tumour cells: R2, macroscopically tumour tissue is left after resection, examined intraoperatively.
- Response Evaluation Criteria for Solid Tumors
-
(RECIST). A classification system used to classify disease response and/or progression over time or in disease response to treatment.
- S1
-
A single-agent chemotherapy.
- Stereotactic body radiotherapy
-
(SBRT). A type of radiation therapy that enables higher and more precise dosage whereby surrounding health tissue is spared.
- Total pancreatectomy
-
Resection of the complete pancreas (that is, pancreatic head, body and tail), at least combined with resection of gallbladder, duodenum, proximal jejunum and, eventually, the distal stomach.
- Upfront surgery
-
Immediate surgery without preoperative therapy with chemotherapy or chemoradiotherapy.
- Volumetric modulated arc therapy
-
A type of radiation therapy that enables higher and more precise dosage whereby surrounding health tissue is spared.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stoop, T.F., Theijse, R.T., Seelen, L.W.F. et al. Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer. Nat Rev Gastroenterol Hepatol 21, 101–124 (2024). https://doi.org/10.1038/s41575-023-00856-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00856-2