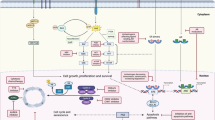
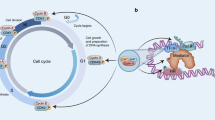
Abstract
Cyclin-dependent kinase (CDK) 4/6 inhibition in combination with endocrine therapy is the standard-of-care treatment for patients with advanced-stage hormone receptor-positive, HER2 non-amplified (HR+HER2−) breast cancer. These agents can also be administered as adjuvant therapy to patients with higher-risk early stage disease. Nonetheless, the clinical success of these agents has created several challenges, such as how to address acquired resistance, identifying which patients are most likely to benefit from therapy prior to treatment, and understanding the optimal timing of administration and sequencing of these agents. In this Review, we describe the rationale for targeting CDK4/6 in patients with breast cancer, including a summary of updated clinical evidence and how this should inform clinical practice. We also discuss ongoing research efforts that are attempting to address the various challenges created by the widespread implementation of these agents.
Key points
-
Cyclin-dependent kinase (CDK) 4/6 inhibitors inhibit the cell cycle, thus inducing cellular senescence and are increasingly being implicated in antitumour immunity.
-
Three CDK4/6 inhibitors are currently used in routine clinical practice and have shown highly consistent progression-free survival results, although inconsistencies have emerged in their overall survival results.
-
The toxicity profile varies between different CDK4/6 inhibitors, allowing a degree of treatment tailoring based on the needs of individual patients.
-
The increased and earlier use of CDK4/6 inhibitors has resulted in a better understanding of the mechanisms of acquired resistance; potential treatment combinations that might overcome these mechanisms are being explored.
-
Treatment selection after disease progression on a CDK4/6 inhibitor remains an area of active research and is likely to be influenced by the underlying mechanisms of resistance
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Beaver, J. A. et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin. Cancer Res. 21, 4760–4766 (2015).
Shah, A. et al. FDA approval: ribociclib for the treatment of postmenopausal women with hormone receptor-positive, HER2-negative advanced or metastatic breast cancer. Clin. Cancer Res. 24, 2999–3004 (2018).
US Food and Drug Administration. FDA approves abemaciclib for HR-positive, HER2-negative breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer (2017).
US Food and Drug Administration. FDA approves abemaciclib with endocrine therapy for early breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-endocrine-therapy-early-breast-cancer (2021).
Hartwell, L. H., Culotti, J., Pringle, J. R. & Reid, B. J. Genetic control of the cell division cycle in yeast. Science 183, 46–51 (1974).
Morgan, D. O. Principles of CDK regulation. Nature 374, 131–134 (1995).
Nurse, P. Finding CDK: linking yeast with humans. Nat. Cell Biol. 14, 776 (2012).
O’Leary, B., Finn, R. S. & Turner, N. C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13, 417–430 (2016).
Asghar, U., Witkiewicz, A. K., Turner, N. C. & Knudsen, E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 14, 130–146 (2015).
Pavletich, N. P. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 287, 821–828 (1999).
Weintraub, S. J., Prater, C. A. & Dean, D. C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature 358, 259–261 (1992).
Hiebert, S. W., Chellappan, S. P., Horowitz, J. M. & Nevins, J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 6, 177–185 (1992).
Sellers, W. R., Rodgers, J. W. & Kaelin, W. G. Jr. A potent transrepression domain in the retinoblastoma protein induces a cell cycle arrest when bound to E2F sites. Proc. Natl Acad. Sci. USA 92, 11544–11548 (1995).
Kato, J., Matsushime, H., Hiebert, S. W., Ewen, M. E. & Sherr, C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7, 331–342 (1993).
Matsushime, H. et al. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell 71, 323–334 (1992).
Meyerson, M. & Harlow, E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell Biol. 14, 2077–2086 (1994).
Goodrich, D. W., Wang, N. P., Qian, Y. W., Lee, E. Y. & Lee, W. H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell 67, 293–302 (1991).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707 (1993).
Hirai, H., Roussel, M. F., Kato, J. Y., Ashmun, R. A. & Sherr, C. J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol. Cell Biol. 15, 2672–2681 (1995).
Chan, F. K., Zhang, J., Cheng, L., Shapiro, D. N. & Winoto, A. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol. Cell Biol. 15, 2682–2688 (1995).
Shapiro, G. I. et al. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 55, 505–509 (1995).
Benedict, W. F. et al. Level of retinoblastoma protein expression correlates with p16 (MTS-1/INK4A/CDKN2) status in bladder cancer. Oncogene 18, 1197–1203 (1999).
Parry, D., Mahony, D., Wills, K. & Lees, E. Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol. Cell Biol. 19, 1775–1783 (1999).
Zerfass-Thome, K. et al. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol. Cell Biol. 17, 407–415 (1997).
Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K. & Elledge, S. J. The P21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805–816 (1993).
Toyoshima, H. & Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78, 67–74 (1994).
Lee, M. H., Reynisdottir, I. & Massague, J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 9, 639–649 (1995).
Guiley, K. Z. et al. p27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. Science 366, eaaw2106 (2019).
Grimmler, M. et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128, 269–280 (2007).
Rane, S. G. et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet 22, 44–52 (1999).
Malumbres, M. et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118, 493–504 (2004).
Rodriguez-Puebla, M. L. et al. Cdk4 deficiency inhibits skin tumor development but does not affect normal keratinocyte proliferation. Am. J. Pathol. 161, 405–411 (2002).
Spencer, S. L. et al. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369–383 (2013).
Asghar, U. S. et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin. Cancer Res. 23, 5561–5572 (2017).
Gillett, C. et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 54, 1812–1817 (1994).
Hunter, T. & Pines, J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell 79, 573–582 (1994).
Kenny, F. S. et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin. Cancer Res. 5, 2069–2076 (1999).
Sherr, C. J. Cancer cell cycles. Science 274, 1672–1677 (1996).
Sutherland, R. L., Prall, O. W., Watts, C. K. & Musgrove, E. A. Estrogen and progestin regulation of cell cycle progression. J. Mammary Gland Biol. Neoplasia 3, 63–72 (1998).
Zwijsen, R. M. et al. CDK-independent activation of estrogen receptor by cyclin D1. Cell 88, 405–415 (1997).
Neuman, E. et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol. Cell Biol. 17, 5338–5347 (1997).
Barretina, J. et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat. Genet 42, 715–721 (2010).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Herschkowitz, J. I., He, X., Fan, C. & Perou, C. M. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 10, R75 (2008).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Deng, J. et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8, 216–233 (2018).
Lelliott, E. J. et al. CDK4/6 inhibition promotes antitumor immunity through the induction of T-cell memory. Cancer Discov. 11, 2582–2601 (2021).
Schaer, D. A. et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 22, 2978–2994 (2018).
Hurvitz, S. A. et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR+/HER2− breast cancer. Clin. Cancer Res. 26, 566–580 (2020).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015).
US Food and Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer (2018).
Xu, B. et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat. Med. 27, 1904–1909 (2021).
NMPA approves AiRuiKang (dalpiciclib) in combination with fulvestrant for the treatment of relapsed/progressed HR+/HER2- advanced breast cancer. 2022-01-03. https://www.hengrui.com/en/media/detail-149.html (2022).
Fry, D. W. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3, 1427–1438 (2004).
Tripathy, D., Bardia, A. & Sellers, W. R. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin. Cancer Res. 23, 3251–3262 (2017).
Gelbert, L. M. et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest. N. Drugs 32, 825–837 (2014).
Lallena, M. J. et al. In-vitro characterization of abemaciclib pharmacology in ER+ breast cancer cell lines [abstract]. Cancer Res. 75 (Suppl. 15), 3101 (2015).
Hafner, M. et al. Multiomics profiling establishes the polypharmacology of FDA-approved CDK4/6 inhibitors and the potential for differential clinical activity. Cell Chem. Biol. 26, 1067–1080.e8 (2019).
Ogata, R., Kishino, E., Saitoh, W., Koike, Y. & Kurebayashi, J. Resistance to cyclin-dependent kinase (CDK) 4/6 inhibitors confers cross-resistance to other CDK inhibitors but not to chemotherapeutic agents in breast cancer cells. Breast Cancer 28, 206–215 (2021).
Raub, T. J. et al. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab. Dispos. 43, 1360–1371 (2015).
Tolaney, S. M. et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin. Cancer Res. 26, 5310–5319 (2020).
Gao, J. J. et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 21, 250–260 (2020).
Wedam, S. et al. FDA approval summary: palbociclib for male patients with metastatic breast cancer. Clin. Cancer Res. 26, 1208–1212 (2020).
US Food and Drug Administration. Highlights of prescribing information: VERZENIO. FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208716s006s007s008lbl.pdf (2017).
Finn, R. S. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 16, 25–35 (2015).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925–1936 (2016).
Cristofanilli, M. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 17, 425–439 (2016).
Turner, N. C. et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 373, 209–219 (2015).
Goetz, M. P. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 35, 3638–3646 (2017).
Sledge, G. W. Jr. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884 (2017).
Dickler, M. N. et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin. Cancer Res. 23, 5218–5224 (2017).
DeMichele, A. et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin. Cancer Res. 21, 995–1001 (2015).
Infante, J. R. et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 22, 5696–5705 (2016).
Hortobagyi, G. N. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 29, 1541–1547 (2018).
Slamon, D. J. et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 36, 2465–2472 (2018).
Slamon, D. J. et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 382, 514–524 (2020).
Tripathy, D. et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 19, 904–915 (2018).
Flaherty, K. T. et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin. Cancer Res. 18, 568–576 (2012).
Schwartz, G. K. et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (schedule 2/1). Br. J. Cancer 104, 1862–1868 (2011).
Patnaik, A. et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 6, 740–753 (2016).
Hortobagyi, G. N. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375, 1738–1748 (2016).
Novartis. Straightforward ECG monitoring. KISQALI https://www.hcp.novartis.com/products/kisqali/metastatic-breast-cancer/safety/ecg-monitoring/ (2023).
Rugo, H. S. et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist 26, e53–e65 (2021).
Johnston, S. et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5, 5 (2019).
Watson, N. W., Wander, S. A., Shatzel, J. J. & Al-Samkari, H. Venous and arterial thrombosis associated with abemaciclib therapy for metastatic breast cancer. Cancer 128, 3224–3232 (2022).
Rugo, H. S. et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann. Oncol. 33, 616–627 (2022).
Zhang, Y., Ma, Z., Sun, X., Feng, X. & An, Z. Interstitial lung disease in patients treated with cyclin-dependent kinase 4/6 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Breast 62, 162–169 (2022).
Slamon, D. J. et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann. Oncol. 32, 1015–1024 (2021).
Hortobagyi, G. N. et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 386, 942–950 (2022).
Lu, Y. S. et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2− advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin. Cancer Res. 28, 851–859 (2022).
Llombart-Cussac, A. et al. Final overall survival analysis of monarch 2: a phase 3 trial of abemaciclib plus fulvestrant in patients with hormone receptor-positive, HER2-negative advanced breast cancer [abstract]. Cancer Res. 83 (Suppl. 5), PD13-11 (2023).
Goetz, M. P. et al. MONARCH 3: interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2-advanced breast cancer (ABC) [abstract LBA15]. Ann. Oncol. 33 (Suppl. 7), S1384 (2022).
Cristofanilli, M. et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2− ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin. Cancer Res. 28, 3433–3442 (2022).
Finn, R. S. et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res. Treat. 183, 419–428 (2020).
Finn, R. S. et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL plus LET) versus placebo plus letrozole (PBO plus LET) in women with estrogen receptor positive/human epidermal growth factor receptor 2-negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2 [abstract]. J. Clin. Oncol. 40 (Suppl. 17), LBA1003 (2022).
Maurer, B., Brandstoetter, T., Kollmann, S., Sexl, V. & Prchal-Murphy, M. Inducible deletion of CDK4 and CDK6 – deciphering CDK4/6 inhibitor effects in the hematopoietic system. Haematologica 106, 2624–2632 (2021).
Lu, Y. S. et al. Primary results from the randomized phase II RIGHT Choice trial of premenopausal patients with aggressive HR+/HER2− advanced breast cancer treated with ribociclib + endocrine therapy vs physician’s choice combination chemotherapy [abstract]. Cancer Res. 85 (Suppl. 5), GS1-10 (2023).
Gennari, A. et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32, 1475–1495 (2021).
Sonke, G. S. et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2−) advanced breast cancer (ABC) [abstract]. J. Clin. Oncol. 41 (Suppl. 17), LBA1000 (2023).
Bidard, F. C. et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J. Clin. Oncol. 40, 3246–3256 (2022).
Juric, D. et al. Alpelisib plus endocrine therapy (ET) in patients with hormone receptor-positive (HR plus), human epidermal growth factor receptor 2-negative (HER2-), PIK3CA-mutated advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDK4/6i): biomarker analyses from the phase II BYLieve study [abstract]. Cancer Res 82 (Suppl. 4), P5-13-03 (2022).
Turner, N. et al. Capivasertib in hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 388, 2058–2070 (2023).
Rugo, H. S. et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 22, 489–498 (2021).
Pan, H. et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017).
Gnant, M. et al. Adjuvant palbociclib for early breast cancer: the PALLAS trial results (ABCSG-42/AFT-05BIG-14-03). J. Clin. Oncol. 40, 282–293 (2022).
Gray, R. G. et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer [abstract]. J. Clin. Oncol. 31 (Suppl. 18), 5 (2013).
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Loibl, S. et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-the Penelope-B trial. J. Clin. Oncol. 39, 1518–1530 (2021).
Johnston, S. R. D. et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J. Clin. Oncol. 38, 3987–3998 (2020).
Johnston, S. R. D. et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 24, 77–90 (2023).
Rugo, H. S., Vitko, A. & Thoele, K. An overview of adverse event (AE) management for patients (pts) receiving abemaciclib [abstract]. J. Clin. Oncol. 40 (Suppl. 28), 239 (2022).
Royce, M. et al. FDA approval summary: abemaciclib with endocrine therapy for high-risk early breast cancer. J. Clin. Oncol. 40, 1155–1162 (2022).
US Food and Drug Administration. FDA D.I.S.C.O. burst edition: FDA approval of Verzenio (abemaciclib) with endocrine therapy for patients with HR-positive, HER2-negative, node-positive, early breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-verzenio-abemaciclib-endocrine-therapy-patients-hr-positive#:~:Text=On%20March%203%2C%202023%2C%20the,at%20high%20risk%20of%20recurrence (2023).
Slamon, D. et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer: primary results from the phase III NATALEE trial [abstract]. J. Clin. Oncol. 41 (Suppl. 17), LBA500 (2023).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377, 122–131 (2017).
Malorni, L. et al. Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann. Oncol. 29, 1748–1754 (2018).
Ribelles, N. et al. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res. 15, R98 (2013).
Cardoso, F. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 31, 1623–1649 (2020).
Cottu, P. et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann. Oncol. 29, 2334–2340 (2018).
Ma, C. X. et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin. Cancer Res. 23, 4055–4065 (2017).
Prat, A. et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 21, 33–43 (2020).
Johnston, S. et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET trial. J. Clin. Oncol. 37, 178–189 (2019).
Khan, Q. J. et al. Letrozole plus ribociclib versus letrozole plus placebo as neoadjuvant therapy for ER plus breast cancer (FELINE trial) [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 505 (2020).
Curigliano, G. et al. Ribociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity study. Breast 28, 191–198 (2016).
Llombart-Cussac, A. et al. Neoadjuvant letrozole plus palbociclib in patients (pts) with hormone receptor (HR)-positive/HER2-negative early breast cancer (EBC) with baseline Ki67 ≥20% and an Oncotype DX Breast Recurrence Score (R) result (RS) ≥18: DxCARTES [abstract]. Cancer Res. 82 (Suppl. 4), P5-16-12 (2022).
Sorlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100, 8418–8423 (2003).
Lehmann, B. D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121, 2750–2767 (2011).
Finn, R. S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77 (2009).
Witkiewicz, A. K., Cox, D. & Knudsen, E. S. CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer 5, 261–272 (2014).
Goel, S. et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29, 255–269 (2016).
Gianni, L. et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. Lancet Oncol. 19, 249–256 (2018).
Gianni, L. et al. Effects of neoadjuvant trastuzumab, pertuzumab and palbociclib on Ki67 in HER2 and ER-positive breast cancer. NPJ Breast Cancer 8, 1 (2022).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012).
Ciruelos, E. et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA trial. Clin. Cancer Res. 26, 5820–5829 (2020).
Tolaney, S. M. et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 21, 763–775 (2020).
André, F. et al. Final overall survival (OS) for abemaciclib plus trastuzumab +/− fulvestrant versus trastuzumab plus chemotherapy in patients with HR+, HER2+ advanced breast cancer (monarcHER): a randomized, open-label, phase II trial [abstract LBA18]. Ann. Oncol. 33 (Suppl. 7), S1386–S1387 (2022).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Rugo, H. S. et al. Abemaciclib in combination with pembrolizumab for HR+, HER2− metastatic breast cancer: phase 1b study. NPJ Breast Cancer 8, 118 (2022).
Yuan, Y. et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur. J. Cancer 154, 11–20 (2021).
Goodman, A. Palbociclib/fulvestrant does not improve progression-free survival after progression on a CDK4/6 inhibitor in metastatic breast cancer. The ASCO Post https://ascopost.com/issues/january-25-2023/palbociclibfulvestrant-does-not-improve-progression-free-survival-after-progression-on-a-cdk46-inhibitor-in-metastatic-breast-cancer/ (2023).
André, F. et al. Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials. J. Clin. Oncol. 38, 1009 (2020).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Knudsen, E. S. et al. Pan-cancer molecular analysis of the RB tumor suppressor pathway. Commun. Biol. 3, 158 (2020).
Safonov, A. M. et al. Allelic dosage of RB1 drives CDK4/6 inhibitor treatment resistance in metastatic breast cancer. J. Clin. Oncol. 40, 1010 (2022).
Razavi, P. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34, 427–438.e6 (2018).
Li, Z. et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell 34, 893–905.e8 (2018).
Finn, R. S. et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naive metastatic breast cancer. Clin. Cancer Res. 26, 110–121 (2020).
Finn, R. S. et al. Treatment effect of palbociclib plus endocrine therapy by prognostic and intrinsic subtype and biomarker analysis in patients with bone-only disease: a joint analysis of PALOMA-2 and PALOMA-3 clinical trials. Breast Cancer Res. Treat. 184, 23–35 (2020).
Prat, A. et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J. Clin. Oncol. 39, 1458–1467 (2021).
Pascual, T. et al. HARMONIA SOLTI-2101/AFT-58: a head-to-head phase III study comparing ribociclib (RIB) and palbociclib (PAL) in patients (pts) with hormone receptor-positive/HER2-negative/HER2-enriched (HR+/HER2-/HER2-E) advanced breast cancer (ABC) [abstract 272TiP]. Ann. Oncol. 33 (Suppl. 7), S662 (2022).
Turner, N. C. et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 37, 1169–1178 (2019).
Dean, J. L., Thangavel, C., McClendon, A. K., Reed, C. A. & Knudsen, E. S. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 29, 4018–4032 (2010).
Sakurikar, N., Thompson, R., Montano, R. & Eastman, A. A subset of cancer cell lines is acutely sensitive to the Chk1 inhibitor MK-8776 as monotherapy due to CDK2 activation in S phase. Oncotarget 7, 1380–1394 (2016).
Chen, X. et al. Cyclin E overexpression sensitizes triple-negative breast cancer to Wee1 kinase inhibition. Clin. Cancer Res. 24, 6594–6610 (2018).
Gallo, D. et al. CCNE1 amplification is synthetic lethal with PKMYT1 kinase inhibition. Nature 604, 749–756 (2022).
De Angelis, C. et al. Activation of the IFN signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER-positive breast cancer. Clin. Cancer Res. 27, 4870–4882 (2021).
Loibl, S. et al. Development and validation of a composite biomarker predictive of palbociclib + endocrine treatment benefit in early breast cancer: PENELOPE-B and PALLAS trials [abstract]. Cancer Res. 83 (Suppl. 5), PD17-05 (2023).
Wander, S. A. et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 10, 1174–1193 (2020).
Herrera-Abreu, M. T. et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res 76, 2301–2313 (2016).
O’Leary, B. et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. ery 8, 1390–1403 (2018).
Condorelli, R. et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 29, 640–645 (2018).
Opyrchal, M. et al. Aurora-A mitotic kinase induces endocrine resistance through down-regulation of ERα expression in initially ERα+ breast cancer cells. PLoS ONE 9, e96995 (2014).
Gong, X. et al. Aurora a kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 9, 248–263 (2019).
Oser, M. G. et al. Cells lacking the RB1 tumor suppressor gene are hyperdependent on aurora B kinase for survival. Cancer Discov. 9, 230–247 (2019).
Haddad, T. C. et al. Evaluation of alisertib alone or combined with fulvestrant in patients with endocrine-resistant advanced breast cancer: the phase 2 TBCRC041 randomized clinical trial. JAMA Oncol. 9, 815–824 (2023).
Soria-Bretones, I. et al. The spindle assembly checkpoint is a therapeutic vulnerability of CDK4/6 inhibitor-resistant ER+ breast cancer with mitotic aberrations. Sci. Adv. 8, eabq4293 (2022).
Yang, C. et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36, 2255–2264 (2017).
Cornell, L., Wander, S. A., Visal, T., Wagle, N. & Shapiro, G. I. MicroRNA-mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Rep. 26, 2667–2680.e7 (2019).
Li, Q. et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 12, 356–371 (2022).
Costa, C. et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov. 10, 72–85 (2020).
Turner, N. C. et al. Capivasertib in hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 388, 2058–2070 (2023).
Turner, N., Sharpe, R., Johnson, D., Pearson, A. & Ashworth, A. FGFR signalling drives the growth of triple negative and basal-like breast cancer cell lines both in vitro and in vivo. Cancer Res. 70, 5275–5286 (2010).
Mao, P. et al. Acquired FGFR and FGF alterations confer resistance to estrogen receptor (ER) targeted therapy in ER+ metastatic breast cancer. Clin. Cancer Res. 26, 5974–5989 (2020).
Formisano, L. et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER plus breast cancer. Nat. Commun. 10, 1373 (2019).
Goetz, M. P. et al. Acquired genomic alterations in circulating tumor DNA from patients receiving abemaciclib alone or in combination with endocrine therapy. J. Clin. Oncol. 38, 3519 (2020).
Pandey, K. et al. Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers 12, 3566 (2020).
Kaklamani, V. et al. Oral elacestrant vs standard-of-care in estrogen receptor-positive, HER2-negative (ER+/HER2-) advanced or metastatic breast cancer (mBC) without detectable ESR1 mutation (EMERALD): Subgroup analysis by prior duration of CDK4/6i plus endocrine therapy (ET). J. Clin. Onc. 41 (no. 16_suppl.) 1070-1070 (2023).
US Food and Drug Administration. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer (2023).
Nayar, U. et al. Acquired HER2 mutations in ER+ metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat. Genet. 51, 207–216 (2019).
Croessmann, S. et al. Combined blockade of activating ERBB2 mutations and ER results in synthetic lethality of ER+/HER2 mutant breast cancer. Clin. Cancer Res. 25, 277–289 (2019).
Bose, R. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 3, 224–237 (2013).
Jhaveri, K. L. et al. Neratinib plus fulvestrant plus trastzuzumab (N+F+T) for hormone receptor-positive (HR+), HER2-negative, HER2-mutant metastatic breast cancer (MBC): outcomes and biomarker analysis from the SUMMIT trial [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 1028 (2022).
Ma, C. X. et al. A phase II trial of neratinib (NER) or NER plus fulvestrant (FUL) (N+F) in HER2 mutant, non-amplified (HER2mut) metastatic breast cancer (MBC): part II of MutHER [abstract]. Cancer Res. 81 (Suppl. 13), CT026 (2021).
Bidard, F. C. et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 23, 1367–1377 (2022).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Kalinsky, K. et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial [abstract]. J. Clin. Oncol. 40 (Suppl. 17), LBA1004 (2022).
Mayer, E. L. et al. Palbociclib after CDK4/6i and endocrine therapy (PACE): a randomised phase II study of fulvestrant, palbociclib and avelumab for endocrine pre-treated ER+/HER2- metastatic breast cancer. 2022 San Antonia Breast Cancer Symposium. Abstract GS3-06. (8 December 2022).
Llombart-Cussac, A. et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR[+])/human epidermal growth factor receptor 2-negative (HER2[−]) advanced breast cancer (ABC): PALMIRA trial [abstract]. J. Clin. Oncol. 41 (Suppl. 16), 1001 (2023).
Wander, S. A. et al. Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: a multicenter experience. J. Natl Compr. Canc. Netw. https://doi.org/10.6004/jnccn.2020.7662 (2021).
Darrigues, L. et al. Circulating tumor DNA as a dynamic biomarker of response to palbociclib and fulvestrant in metastatic breast cancer patients. Breast Cancer Res. 23, 31 (2021).
Yap, T. A. et al. First-in-human first-in-class phase 1/2a study of the next generation CDK4-selective inhibitor PF-07220060 in patients (pts) with advanced solid tumors, enriched for HR+ HER2− mBC who progressed on prior CDK4/6 inhibitors and endocrine therapy [abstract]. J. Clin. Oncol. 41 (Suppl. 16), 3009 (2023).
Rizzolio, F., Tuccinardi, T., Caligiuri, I., Lucchetti, C. & Giordano, A. CDK inhibitors: from the bench to clinical trials. Curr. Drug Targets 11, 279–290 (2010).
Yap, T. et al. Abstract P5-16-06: A first-in-human phase 1/2a dose escalation/expansion study of the first-in-class CDK2/4/6 inhibitor PF-06873600 alone or in combination with endocrine therapy in patients with breast or ovarian cancer. Cancer Res. 82 (Suppl. 4), P5-16-06 (2022).
Brown, V. et al. Abstract 2306: BLU-222, an investigational, potent, and selective CDK2 inhibitor, demonstrated robust antitumor activity in CCNE1-amplified ovarian cancer models. Cancer Res. 82 (Suppl. 12), 2306 (2022).
Choi, Y. J. et al. Development of a selective CDK2-E inhibitor in CCNE driven cancers [abstract]. Cancer Res 81 (Suppl. 13), 1279 (2021).
Vora, S. R. et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 26, 136–149 (2014).
O’Brien, N. A. et al. Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res. 22, 89 (2020).
Tolaney, S. M. et al. Phase Ib study of ribociclib plus fulvestrant and ribociclib plus fulvestrant plus PI3K inhibitor (alpelisib or buparlisib) for HR+ advanced breast cancer. Clin. Cancer Res. 27, 418–428 (2021).
Author information
Authors and Affiliations
Contributions
L.M. and N.C.T. researched data for the manuscript and L.M. wrote the manuscript. All authors made a substantial contribution to discussions of content and edited and/or reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
L.M. and S.L. declare no competing interests. N.C.T. has acted as an adviser to Astra Zeneca, Exact Sciences, Gilead, GlaxoSmithKline, Guardant, Inivata, Lilly, Novartis, Pfizer, Relay therapeutics, Repare therapeutics, Roche/Genentech and Zentalis, and has received research funding from Astra Zeneca, Guardant Health, Inivata, Invitae, Merck Sharpe and Dohme, Natera, Personalis, Pfizer and Roche/Genentech.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks C.L. Arteaga and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morrison, L., Loibl, S. & Turner, N.C. The CDK4/6 inhibitor revolution — a game-changing era for breast cancer treatment. Nat Rev Clin Oncol 21, 89–105 (2024). https://doi.org/10.1038/s41571-023-00840-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00840-4