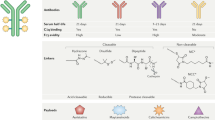
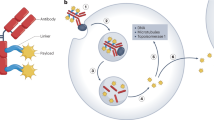
Abstract
Over the past 5 years, improvements in the design of antibody–drug conjugates (ADCs) have enabled major advances that have reshaped the treatment of several advanced-stage solid tumours. Considering the intended rationale behind the design of ADCs, which is to achieve targeted delivery of cytotoxic molecules by linking them to antibodies targeting tumour-specific antigens, ADCs would be expected to be less toxic than conventional chemotherapy. However, most ADCs are still burdened by off-target toxicities that resemble those of the cytotoxic payload as well as on-target toxicities and other poorly understood and potentially life-threatening adverse effects. Given the rapid expansion in the clinical indications of ADCs, including use in curative settings and various combinations, extensive efforts are ongoing to improve their safety. Approaches currently being pursued include clinical trials optimizing the dose and treatment schedule, modifications of each ADC component, identification of predictive biomarkers for toxicities, and the development of innovative diagnostic tools. In this Review, we describe the determinants of the toxicities of ADCs in patients with solid tumours, highlighting key strategies that are expected to improve tolerability and enable improvements in the treatment outcomes of patients with advanced-stage and those with early stage cancers in the years to come.
Key points
-
Indications for the use of antibody–drug conjugates (ADCs) are rapidly expanding, with development progressively moving from the advanced-stage to the early stage setting, and from monotherapy to combination strategies.
-
Despite being designed with the rationale of expanding the therapeutic indices of conventional chemotherapies, most ADCs have a toxicity profile similar to that of their cytotoxic payload.
-
Unconventional and potentially life-threatening toxicities can also be observed with certain ADCs, requiring an increased understanding of these events and the optimization of diagnostic and management practices.
-
Multiple pharmacological modification strategies are being pursued in an attempt to improve the tolerability of ADCs, including molecular alterations of the antibody moiety, linker and/or cytotoxic payload.
-
Exploration of different doses within randomized trials and investigation of response-adapted dosing strategies could enable optimized use of ADCs, which could maximize their therapeutic value for each indication.
-
Extensive efforts to identify biomarkers of toxicities in patients receiving ADCs and to develop diagnostic tools enabling the anticipation and/or early detection of toxicities are currently ongoing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tarantino, P. et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J. Clin. https://doi.org/10.3322/caac.21705 (2021).
Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal. Transduct. Target. Ther. 7, 93 (2022).
de la Torre, B. G. & Albericio, F. The pharmaceutical industry in 2022: an analysis of FDA drug approvals from the perspective of molecules. Molecules 28, 1038 (2023).
do Pazo, C., Nawaz, K. & Webster, R. M. The oncology market for antibody-drug conjugates. Nat. Rev. Drug Discov. 20, 583–584 (2021).
Modi, S. et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J. Clin. Oncol. 38, 1887–1896 (2020).
Banerji, U. et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20, 1124–1135 (2019).
Wang, J. et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: a pooled analysis of two studies. J. Clin. Oncol. 39, 1022–1022 (2021).
Schmid, P. et al. BEGONIA: phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC) — initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J. Clin. Oncol. 39, 1023–1023 (2021).
Jiang, Z. et al. A multiple center, open-label, single-arm, phase II clinical trial of MRG002, an HER2-targeted antibody-drug conjugate, in patients with HER2-low expressing advanced or metastatic breast cancer. J. Clin. Oncol. 40, 1102–1102 (2022).
Yamaguchi, K. et al. Trastuzumab deruxtecan in anti–-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2-low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J. Clin. Oncol. 41, 816–825 (2023).
Tarantino, P. et al. HER2-low breast cancer: pathological and clinical landscape. J. Clin. Oncol. 38, 1951–1962 (2020).
AstrazZeneca. Enhertu Showed Clinically Meaningful and Durable Responses Across Multiple HER2-Expressing Tumour Types in DESTINY-PanTumor02 Phase II Trial https://www.astrazeneca.com/media-centre/press-releases/2023/enhertu-destiny-pantumor02-shows-positive-results.html (2023).
Drago, J. Z., Modi, S. & Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 18, 327–344 (2021).
Colombo, R. & Rich, J. R. The therapeutic window of antibody drug conjugates: a dogma in need of revision. Cancer Cell 40, 1255–1263 (2022).
Zhu, Y., Liu, K., Wang, K. & Zhu, H. Treatment-related adverse events of antibody-drug conjugates in clinical trials: a systematic review and meta-analysis. Cancer 129, 283–295 (2023).
Blackhall, F. et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J. Thorac. Oncol. 16, 1547–1558 (2021).
Lassman, A. B. et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro-Oncology 25, 339–350 (2022).
Junttila, T. T., Li, G., Parsons, K., Phillips, G. L. & Sliwkowski, M. X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 128, 347–356 (2011).
Chang, H.-P., Le, H. K. & Shah, D. K. Pharmacokinetics and pharmacodynamics of antibody-drug conjugates administered via subcutaneous and intratumoral routes. Pharmaceutics 15, 1132 (2023).
Jin, Y., Schladetsch, M. A., Huang, X., Balunas, M. J. & Wiemer, A. J. Stepping forward in antibody-drug conjugate development. Pharmacol. Ther. 229, 107917 (2022).
Epenetos, A. A., Snook, D., Durbin, H., Johnson, P. M. & Taylor-Papadimitriou, J. Limitations of radiolabeled monoclonal antibodies for localization of human neoplasms. Cancer Res. 46, 3183–3191 (1986).
Mach, J.-P. et al. Tumor localization of radio-labeled antibodies against carcinoembryonic antigen in patients with carcinoma. N. Engl. J. Med. 303, 5–10 (1980).
Marei, H. E., Cenciarelli, C. & Hasan, A. Potential of antibody–drug conjugates (ADCs) for cancer therapy. Cancer Cell Int. 22, 255 (2022).
Mahalingaiah, P. K. et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol. Ther. 200, 110–125 (2019).
Giugliano, F., Corti, C., Tarantino, P., Michelini, F. & Curigliano, G. Bystander effect of antibody-drug conjugates: fact or fiction? Curr. Oncol. Rep. 24, 809–817 (2022).
Khera, E. et al. Cellular-resolution imaging of bystander payload tissue penetration from antibody-drug conjugates. Mol. Cancer Ther. 21, 310–321 (2022).
Suzuki, M. et al. Visualization of intratumor pharmacokinetics of [fam-] trastuzumab deruxtecan (DS-8201a) in HER2 heterogeneous model using phosphor-integrated dots imaging analysis. Clin. Cancer Res. 27, 3970–3979 (2021).
Okeley, N. M. et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin. Cancer Res. 16, 888–897 (2010).
Staudacher, A. H. & Brown, M. P. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br. J. Cancer 117, 1736–1742 (2017).
Kopp, A. et al. Antibody–drug conjugate sacituzumab govitecan drives efficient tissue penetration and rapid intracellular drug release. Mol. Cancer Ther. 22, 102–111 (2023).
Lucas, A. T. et al. Factors affecting the pharmacology of antibody-drug conjugates. Antibodies https://doi.org/10.3390/antib7010010 (2018).
Han, T. H. & Zhao, B. Absorption, distribution, metabolism, and excretion considerations for the development of antibody-drug conjugates. Drug Metab. Dispos. 42, 1914–1920 (2014).
Wei, C. et al. Where did the linker-payload go? A quantitative investigation on the destination of the released linker-payload from an antibody-drug conjugate with a maleimide linker in plasma. Anal. Chem. 88, 4979–4986 (2016).
Nilsen, J. et al. Human and mouse albumin bind their respective neonatal Fc receptors differently. Sci. Rep. 8, 14648 (2018).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Polson, A. G. et al. Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: target and linker-drug selection. Cancer Res. 69, 2358–2364 (2009).
Hamblett, K. J. et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 10, 7063–7070 (2004).
Sun, X. et al. Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-maytansinoid conjugates. Bioconjug. Chem. 28, 1371–1381 (2017).
Nguyen, T. D., Bordeau, B. M. & Balthasar, J. P. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers 15, 713 (2023).
Powles, T. et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 384, 1125–1135 (2021).
Pitot, H. C. et al. Phase I trial of dolastatin-10 (NSC 376128) in patients with advanced solid tumors. Clin. Cancer Res. 5, 525–531 (1999).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Franklin, R. et al. A phase I-II study of maytansine utilizing a weekly schedule. Cancer 46, 1104–1108 (1980).
Eagan, R. T., Ingle, J. N., Rubin, J., Frytak, S. & Moertel, C. G. Early clinical study of an intermittent schedule for maytansine (NSC-153858): brief communication. J. Natl Cancer Inst. 60, 93–96 (1978).
Bardia, A. et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 384, 1529–1541 (2021).
Spring, L. et al. Phase 2 study of response-guided neoadjuvant sacituzumab govitecan (IMMU-132) in patients with localized triple-negative breast cancer: results from the NeoSTAR trial. J. Clin. Oncol. 40, 512–512 (2022).
Rougier, P. et al. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J. Clin. Oncol. 15, 251–260 (1997).
Conilh, L., Sadilkova, L., Viricel, W. & Dumontet, C. Payload diversification: a key step in the development of antibody–drug conjugates. J. Hematol. Oncol. 16, 3 (2023).
Colombo, R., Barnscher, S. D. & Rich, J. R. Abstract 1538: revisiting the dogma of antibody drug conjugates (ADCs): emerging data challenge the benefit of linker stability and the primacy of payload delivery. Cancer Res. 83, 1538–1538 (2023).
Donaghy, H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. mAbs 8, 659–671 (2016).
Bargh, J. D., Isidro-Llobet, A., Parker, J. S. & Spring, D. R. Cleavable linkers in antibody-drug conjugates. Chem. Soc. Rev. 48, 4361–4374 (2019).
Tarcsa, E., Guffroy, M. R., Falahatpisheh, H., Phipps, C. & Kalvass, J. C. Antibody-drug conjugates as targeted therapies: are we there yet? A critical review of the current clinical landscape. Drug Discov. Today Technol. 37, 13–22 (2020).
Zhang, J. et al. Phase I study of A166, an antibody–drug conjugate in advanced HER2-expressing solid tumours. NPJ Breast Cancer 9, 28 (2023).
Zhang, J. et al. Phase I trial of a novel anti-HER2 antibody–drug conjugate, ARX788, for the treatment of HER2-positive metastatic breast cancer. Clin. Cancer Res. 28, 4212–4221 (2022).
Saura Manich, C. et al. LBA15 Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 32, S1288 (2021).
Matsuda, Y. & Mendelsohn, B. A. An overview of process development for antibody-drug conjugates produced by chemical conjugation technology. Expert Opin. Biol. Ther. 21, 963–975 (2021).
Saleh, M. N. et al. Phase I trial of the anti-Lewis Y drug immunoconjugate BR96-doxorubicin in patients with Lewis Y-expressing epithelial tumors. J. Clin. Oncol. 18, 2282–2292 (2000).
Fu, Z., Liu, J., Li, S., Shi, C. & Zhang, Y. Treatment-related adverse events associated with HER2-targeted antibody-drug conjugates in clinical trials: a systematic review and meta-analysis. eClinicalMedicine https://doi.org/10.1016/j.eclinm.2022.101795 (2023).
Krop, I. et al. Abstract GS1-05: Datopotamab deruxtecan in advanced/metastatic HER2- breast cancer: results from the phase 1 TROPION-PanTumor01 study. Cancer Res. https://doi.org/10.1158/1538-7445.Sabcs21-gs1-05 (2022).
King, G. T. et al. A phase 1, dose-escalation study of PF-06664178, an anti-Trop-2/Aur0101 antibody-drug conjugate in patients with advanced or metastatic solid tumors. Invest. N. Drugs 36, 836–847 (2018).
Stepan, L. P. et al. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J. Histochem. Cytochem. 59, 701–710 (2011).
Krop, I. E. et al. Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC). J. Clin. Oncol. 40, 1002–1002 (2022).
Wakui, H. et al. Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 73, 511–516 (2014).
Coleman, R. L. et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 22, 609–619 (2021).
Shi, F. et al. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. 29, 1335–1344 (2022).
Challita-Eid, P. M. et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76, 3003–3013 (2016).
Unruh, D. & Horbinski, C. Beyond thrombosis: the impact of tissue factor signaling in cancer. J. Hematol. Oncol. 13, 93 (2020).
Annunziata, C. M. et al. Phase 1, open-label study of MEDI-547 in patients with relapsed or refractory solid tumors. Invest. N. Drugs 31, 77–84 (2013).
Boyd, A. W., Bartlett, P. F. & Lackmann, M. Therapeutic targeting of EPH receptors and their ligands. Nat. Rev. Drug Discov. 13, 39–62 (2014).
Schettini, F. et al. Identification of cell surface targets for CAR-T cell therapies and antibody-drug conjugates in breast cancer. ESMO Open 6, 100102 (2021).
Kumagai, K. et al. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 111, 4636–4645 (2020).
Berger, M. et al. Tissue-specific Fc gamma and complement receptor expression by alveolar macrophages determines relative importance of IgG and complement in promoting phagocytosis of Pseudomonas aeruginosa. Pediatr. Res. 35, 68–77 (1994).
Bruggeman, C. W. et al. Tissue-specific expression of IgG receptors by human macrophages ex vivo. PLoS One 14, e0223264 (2019).
Tarantino, P. et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. 7, 1873–1881 (2021).
Uppal, H. et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin. Cancer Res. 21, 123–133 (2015).
Ansary, A. M. et al. Effect of Ado-trastuzumab emtansine on autologous platelet kinetics and function. JCO Precis. Oncol. 6, e2200237 (2022).
Powell, C. A. et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 7, 100554 (2022).
Tang, M. et al. Clinical pharmacology of the antibody-drug conjugate enfortumab vedotin in advanced urothelial carcinoma and other malignant solid tumors. J. Clin. Oncol. 40, 568–568 (2022).
Bardia, A. et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 32, 746–756 (2021).
Rugo, H. S. et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer 8, 98 (2022).
Yin, O. et al. Population pharmacokinetics of trastuzumab deruxtecan in patients with HER2-positive breast cancer and other solid tumors. Clin. Pharmacol. Ther. 109, 1314–1325 (2021).
Gibiansky, L. et al. Population pharmacokinetic analysis for tisotumab vedotin in patients with locally advanced and/or metastatic solid tumors. CPT Pharmacomet. Syst. Pharmacol. 11, 1358–1370 (2022).
Moore, K. N. et al. 605P Population pharmacokinetic (PK) analysis of mirvetuximab soravtansine (MIRV) in patients with folate receptor α (FRα)-positive cancer. Ann. Oncol. 33, S822–S823 (2022).
Lu, D. et al. Population pharmacokinetics of trastuzumab emtansine (T-DM1), a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer: clinical implications of the effect of covariates. Cancer Chemother. Pharmacol. 74, 399–410 (2014).
Yardley, D. A. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int. J. Breast Cancer 2013, 137414 (2013).
Kan, S. et al. Up-regulation of HER2 by gemcitabine enhances the antitumor effect of combined gemcitabine and trastuzumab emtansine treatment on pancreatic ductal adenocarcinoma cells. BMC Cancer 15, 726 (2015).
Martin, M. et al. Trastuzumab emtansine (T-DM1) plus docetaxel with or without pertuzumab in patients with HER2-positive locally advanced or metastatic breast cancer: results from a phase Ib/IIa study. Ann. Oncol. 27, 1249–1256 (2016).
Cortés, J. et al. Efficacy and safety of trastuzumab emtansine plus capecitabine vs trastuzumab emtansine alone in patients with previously treated ERBB2 (HER2)-positive metastatic breast cancer: a phase 1 and randomized phase 2 trial. JAMA Oncol. 6, 1203–1209 (2020).
Janjigian, Y. Y. et al. Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients (pts) with advanced/metastatic HER2+ gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. J. Clin. Oncol. 40, 295–295 (2022).
Levy, B. et al. MA13.07 TROPION-Lung02: initial results for datopotamab deruxtecan plus pembrolizumab and platinum chemotherapy in advanced NSCLC. J. Thorac. Oncol. 17, S91 (2022).
Moore, K. N. et al. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol. Oncol. 151, 46–52 (2018).
Risbridger, G. P., Davis, I. D., Birrell, S. N. & Tilley, W. D. Breast and prostate cancer: more similar than different. Nat. Rev. Cancer 10, 205–212 (2010).
Mamounas, E. P. et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: subgroup analyses from KATHERINE. Ann. Oncol. 32, 1005–1014 (2021).
Hurvitz, S. A. et al. TRIO-US B-12 TALENT: Phase II neoadjuvant trial evaluating trastuzumab deruxtecan with or without anastrozole for HER2-low, HR+ early stage breast cancer. J. Clin. Oncol. 40 (Suppl. 16), TPS623 (2022).
Andre, F. et al. Dose-finding and -expansion studies of trastuzumab deruxtecan in combination with other anti-cancer agents in patients (pts) with advanced/metastatic HER2+ (DESTINY-Breast07 [DB-07]) and HER2-low (DESTINY-Breast08 [DB-08]) breast cancer (BC).J. Clin. Oncol. 40, 3025 (2022).
Galluzzi, L., Humeau, J., Buqué, A., Zitvogel, L. & Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 17, 725–741 (2020).
Bauzon, M. et al. Maytansine-bearing antibody-drug conjugates induce in vitro hallmarks of immunogenic cell death selectively in antigen-positive target cells. OncoImmunology 8, e1565859 (2019).
Cao, A., Heiser, R., Law, C.-L. & Gardai, S. J. Abstract 4914: Auristatin-based antibody drug conjugates activate multiple ER stress response pathways resulting in immunogenic cell death and amplified T-cell responses. Cancer Res. 76, 4914–4914 (2016).
Hasan, M. M., Laws, M., Jin, P. & Rahman, K. M. Factors influencing the choice of monoclonal antibodies for antibody–drug conjugates. Drug Discov. Today 27, 354–361 (2022).
Nicolò, E. et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treat. Rev. 106, 102395 (2022).
Spain, L., Diem, S. & Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 44, 51–60 (2016).
Emens, L. A. et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 21, 1283–1295 (2020).
Rosenberg, J. E. et al. LBA73 — Study EV-103 Cohort K: antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC). Ann. Oncol. 33 (Suppl. 7), S1441 (2022).
Hamilton, E. et al. Abstract PD3-07: Trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: a 2-part, phase 1b, multicenter, open-label study. Cancer Res. https://doi.org/10.1158/1538-7445.Sabcs20-pd3-07 (2021).
Galsky, M. D. et al. Primary analysis from DS8201-A-U105: A phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC). J. Clin. Oncol. 40, 438–438 (2022).
Schmid, P., Jung, K. H., Wysocki, P. J., Jassem, J. & Ma, C. 166MO — Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): initial results from BEGONIA, a phase 1b/2 study. Ann. Oncol. 33, S199 (2022).
Grivas, P. et al. TROPHY-U-01 Cohort 3: sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) who progressed after platinum (PLT)-based regimens. J. Clin. Oncol. 40, 434–434 (2022).
Schmid, P. et al. Abstract PD11-08: PD11-08 Trastuzumab deruxtecan (T-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic hormone receptor-negative (HR−), HER2-low breast cancer: updated results from BEGONIA, a phase 1b/2 study. Cancer Res. 83, PD11-08-PD11-08 (2023).
Waks, A. G. et al. Phase Ib study of pembrolizumab in combination with trastuzumab emtansine for metastatic HER2-positive breast cancer. J. Immunother. Cancer https://doi.org/10.1136/jitc-2022-005119 (2022).
Borges, V. F. et al. Tucatinib combined with Ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 4, 1214–1220 (2018).
Spring, L. M. et al. Phase 1b clinical trial of ado-trastuzumab emtansine and ribociclib for HER2-positive metastatic breast cancer. NPJ Breast Cancer 7, 103 (2021).
Bardia, A. et al. Abstract 2638: Sacituzumab govitecan, combination with PARP inhibitor, talazoparib, in metastatic triple-negative breast cancer (TNBC): translational investigation. Cancer Res. 82, 2638–2638 (2022).
O’Malley, D. M. et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 157, 379–385 (2020).
Matulonis, U. A. et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J. Clin. Oncol. 41, 2436–2445 (2023).
Liao, M. Z. et al. Model-informed therapeutic dose optimization strategies for antibody-drug conjugates in oncology: what can we learn from US Food and Drug Administration-approved antibody-drug conjugates? Clin. Pharmacol. Ther. 110, 1216–1230 (2021).
Hanna, K. S. Clinical overview of enfortumab vedotin in the management of locally advanced or metastatic urothelial carcinoma. Drugs 80, 1–7 (2020).
Moore, K. N. et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors. Cancer 123, 3080–3087 (2017).
Rivera, E. & Cianfrocca, M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother. Pharmacol. 75, 659–670 (2015).
Iveson, T. et al. Prospective pooled analysis of four randomized trials investigating duration of adjuvant (adj) oxaliplatin-based therapy (3 vs 6 months {m}) for patients (pts) with high-risk stage II colorectal cancer (CC). J. Clin. Oncol. 37, 3501–3501 (2019).
Lu, D. et al. Time-to-event analysis of polatuzumab vedotin-induced peripheral neuropathy to assist in the comparison of clinical dosing regimens. CPT Pharmacomet. Syst. Pharmacol. 6, 401–408 (2017).
Deeks, E. D. Polatuzumab vedotin: first global approval. Drugs 79, 1467–1475 (2019).
Norsworthy, K. J. et al. FDA approval summary: mylotarg for treatment of patients with relapsed or refractory CD33-positive acute myeloid leukemia. Oncologist 23, 1103–1108 (2018).
Advani, A. et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J. Clin. Oncol. 28, 2085–2093 (2010).
Kantarjian, H. et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer 119, 2728–2736 (2013).
Kantarjian, H. M. et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N. Engl. J. Med. 375, 740–753 (2016).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401, 105–117 (2023).
Tarantino, P. et al. Aiming at a tailored cure for ERBB2-positive metastatic breast cancer: a review. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2021.6597 (2022).
Tamura, K. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 20, 816–826 (2019).
Goto, K. et al. LBA55 Trastuzumab deruxtecan (T-DXd) in patients (Pts) with HER2-mutant metastatic non-small cell lung cancer (NSCLC): interim results from the phase 2 DESTINY-Lung02 trial. Ann. Oncol. 33, S1422 (2022).
Autio, K. A., Boni, V., Humphrey, R. W. & Naing, A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin. Cancer Res. 26, 984–989 (2020).
Bordeau, B. M., Yang, Y. & Balthasar, J. P. Transient competitive inhibition bypasses the binding site barrier to improve tumor penetration of trastuzumab and enhance T-DM1 efficacy. Cancer Res. 81, 4145–4154 (2021).
Chen, P., Bordeau, B. M., Zhang, Y. & Balthasar, J. P. Transient inhibition of trastuzumab–tumor binding to overcome the “binding-site barrier” and improve the efficacy of a trastuzumab–gelonin immunotoxin. Mol. Cancer Ther. 21, 1573–1582 (2022).
Boni, V. et al. Praluzatamab ravtansine, a CD166-targeting antibody–drug conjugate, in patients with advanced solid tumors: an open-label phase I/II trial. Clin. Cancer Res. 28, 2020–2029 (2022).
Aoyama, M., Tada, M., Yokoo, H., Demizu, Y. & Ishii-Watabe, A. Fcγ receptor-dependent internalization and off-target cytotoxicity of antibody-drug conjugate aggregates. Pharm. Res. 39, 89–103 (2022).
Scribner, J. A. et al. Preclinical evaluation of IMGC936, a next-generation maytansinoid-based antibody–drug conjugate targeting ADAM9-expressing tumors. Mol. Cancer Ther. 21, 1047–1059 (2022).
Mazor, Y. et al. Improving target cell specificity using a novel monovalent bispecific IgG design. mAbs 7, 377–389 (2015).
Sellmann, C. et al. Balancing selectivity and efficacy of bispecific epidermal growth factor receptor (EGFR) × c-MET antibodies and antibody-drug conjugates. J. Biol. Chem. 291, 25106–25119 (2016).
Hamblett, K. J. et al. Abstract 3914: ZW49, a HER2-targeted biparatopic antibody-drug conjugate for the treatment of HER2-expressing cancers. Cancer Res. 78, 3914–3914 (2018).
Antonarelli, G. et al. Research and clinical landscape of bispecific antibodies for the treatment of solid malignancies. Pharmaceuticals https://doi.org/10.3390/ph14090884 (2021).
Jhaveri, K. et al. 460MO Preliminary results from a phase I study using the bispecific, human epidermal growth factor 2 (HER2)-targeting antibody-drug conjugate (ADC) zanidatamab zovodotin (ZW49) in solid cancers. Ann. Oncol. 33, S749–S750 (2022).
Yamaguchi, A. et al. Chemical generation of small molecule-based bispecific antibody-drug conjugates for broadening the target scope. Bioorg. Med. Chem. 32, 116013 (2021).
de Goeij, B. E. et al. Efficient payload delivery by a bispecific antibody-drug conjugate targeting HER2 and CD63. Mol. Cancer Ther. 15, 2688–2697 (2016).
Andreev, J. et al. Bispecific antibodies and antibody-drug conjugates (ADCs) bridging HER2 and prolactin receptor improve efficacy of HER2 ADCs. Mol. Cancer Ther. 16, 681–693 (2017).
Hong, Y., Nam, S.-M. & Moon, A. Antibody–drug conjugates and bispecific antibodies targeting cancers: applications of click chemistry. Arch. Pharm. Res. 46, 131–148 (2023).
Shang, C. et al. Abstract 4256: YH012, a novel bispecific anti-HER2 and TROP2 antibody-drug conjugate, exhibits potent antitumor efficacy. Cancer Res. 82, 4256–4256 (2022).
Coumans, R. G. E. et al. A platform for the generation of site-specific antibody–drug conjugates that allows for selective reduction of engineered cysteines. Bioconjugate Chem. 31, 2136–2146 (2020).
Sussman, D. et al. Engineered cysteine antibodies: an improved antibody-drug conjugate platform with a novel mechanism of drug-linker stability. Protein Eng. Des. Sel. 31, 47–54 (2018).
Zhou, Q. et al. Site-specific antibody conjugation to engineered double cysteine residues. Pharmaceuticals https://doi.org/10.3390/ph14070672 (2021).
Tian, F. et al. A general approach to site-specific antibody drug conjugates. Proc. Natl Acad. Sci. USA 111, 1766–1771 (2014).
Nagaraja Shastri, P. et al. Nonclinical development of next-generation site-specific HER2-targeting antibody-drug conjugate (ARX788) for breast cancer treatment. Mol. Cancer Ther. 19, 1822–1832 (2020).
Zhou, Q. Site-specific antibody conjugation for ADC and beyond. Biomedicines https://doi.org/10.3390/biomedicines5040064 (2017).
Simmons, J. K., Burke, P. J., Cochran, J. H., Pittman, P. G. & Lyon, R. P. Reducing the antigen-independent toxicity of antibody-drug conjugates by minimizing their non-specific clearance through PEGylation. Toxicol. Appl. Pharmacol. 392, 114932 (2020).
Liu, S. et al. Abstract 4263: A novel pegylated biparatopic antibody-drug conjugate (pb-adc) targeting cancers with low HER2+ expression. Cancer Res. 82, 4263–4263 (2022).
Shao, T. et al. Construction of paclitaxel-based antibody–drug conjugates with a PEGylated linker to achieve superior therapeutic index. Signal. Transduct. Target. Ther. 5, 132 (2020).
Walker, J. A. et al. Hydrophilic sequence-defined cross-linkers for antibody–drug conjugates. Bioconjugate Chem. 30, 2982–2988 (2019).
Yamazaki, C. M. et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat. Commun. 12, 3528 (2021).
Świderska, K. W., Szlachcic, A., Opaliński, Ł., Zakrzewska, M. & Otlewski, J. FGF2 dual warhead conjugate with monomethyl Auristatin E and α-amanitin displays a cytotoxic effect towards cancer cells overproducing FGF receptor 1. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19072098 (2018).
Nilchan, N. et al. Dual-mechanistic antibody-drug conjugate via site-specific selenocysteine/cysteine conjugation. Antib. Ther. 2, 71–78 (2019).
Kumar, A. et al. Synthesis of a heterotrifunctional linker for the site-specific preparation of antibody-drug conjugates with two distinct warheads. Bioorg. Med. Chem. Lett. 28, 3617–3621 (2018).
Janku, F. et al. Preclinical characterization and phase I study of an anti-HER2-TLR7 immune-stimulator antibody conjugate in patients with HER2+ malignancies. Cancer Immunol. Res. 10, 1441–1461 (2022).
Mersana Therapeutics. Mersana Therapeutics Announces Clinical Hold on XMT-2056 Phase 1 Clinical Trial https://ir.mersana.com/news-releases/news-release-details/mersana-therapeutics-announces-clinical-hold-xmt-2056-phase-1#:~:Text=This%20action%20follows%20the%20company’s,its%20cause%20remain%20under%20investigation (13 March 2023).
Bordeau, B. M., Nguyen, T. D., Polli, J. R., Chen, P. & Balthasar, J. P. Payload-binding Fab fragments increase the therapeutic index of MMAE antibody–drug conjugates. Mol. Cancer Ther. 22, 459–470 (2023).
Toffoli, G. et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J. Clin. Oncol. 24, 3061–3068 (2006).
Liu, X., Cheng, D., Kuang, Q., Liu, G. & Xu, W. Association of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: a meta-analysis in Caucasians. Pharmacogenomics J. 14, 120–129 (2014).
Kweekel, D. M. et al. UGT1A1*28 genotype and irinotecan dosage in patients with metastatic colorectal cancer: a Dutch Colorectal Cancer Group study. Br. J. Cancer 99, 275–282 (2008).
Wijsenbeek, M. S. et al. Home monitoring in interstitial lung diseases. Lancet Respir. Med. 11, 97–110 (2023).
Broos, C. E. et al. Daily home spirometry to detect early steroid treatment effects in newly treated pulmonary sarcoidosis. Eur. Respir. J. 51, 1702089 (2018).
Moor, C. C. et al. Diurnal variation in forced vital capacity in patients with fibrotic interstitial lung disease using home spirometry. ERJ Open Res. https://doi.org/10.1183/23120541.00054-2020 (2020).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628 (2018).
Pondé, N. F., Zardavas, D. & Piccart, M. Progress in adjuvant systemic therapy for breast cancer. Nat. Rev. Clin. Oncol. 16, 27–44 (2019).
Braun, M. S. & Seymour, M. T. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther. Adv. Med. Oncol. 3, 43–52 (2011).
Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32, 1475–1495 (2021).
Giordano, S. H. et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO Guideline update. J. Clin. Oncol. 40, 2612–2635 (2022).
Marmé, F. et al. 58O - Safety interim analysis (SIA) of the phase III postneoadjuvant SASCIA study evaluating sacituzumab govitecan (SG) in patients with primary HER2-negative breast cancer (BC) at high relapse risk after neoadjuvant treatment. Ann. Oncol. 33, S148–S149 (2022).
Hurvitz, S. A. et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 19, 115–126 (2018).
Petrylak, D. P. et al. Study EV-103 cohort H: antitumor activity of neoadjuvant treatment with enfortumab vedotin monotherapy in patients (pts) with muscle invasive bladder cancer (MIBC) who are cisplatin-ineligible. J. Clin. Oncol. 40, 435–435 (2022).
Schmitz, S., Duhoux, F. & Machiels, J. P. Window of opportunity studies: do they fulfil our expectations? Cancer Treat. Rev. 43, 50–57 (2016).
Prat, A. et al. LBA3 Patritumab deruxtecan (HER3-DXd) in early-stage HR+/HER2- breast cancer: final results of the SOLTI TOT-HER3 window of opportunity trial. Ann. Oncol. 33, S164 (2022).
Tarantino, P., et al. Abstract PD18-01: Adjuvant trastuzumab emtansine versus paclitaxel plus trastuzumab for stage I HER2+ breast cancer: 5-year results and correlative analyses from ATEMPT (TBCRC033). Cancer Res. 83, PD18-01-PD18-01 (2023).
Tolaney, S. M. et al. Adjuvant trastuzumab emtansine versus paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT): a randomized clinical trial. J. Clin. Oncol. 39, 2375–2385 (2021).
Barroso-Sousa, R. et al. Cardiac outcomes of subjects on adjuvant trastuzumab emtansine vs paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT) study (TBCRC033): a randomized controlled trial. NPJ Breast Cancer 8, 18 (2022).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Hellmann, M. D. et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 15, e42–e50 (2014).
Menzies, A. M. et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 27, 301–309 (2021).
Meredith, K. L. et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann. Surg. Oncol. 17, 1159–1167 (2010).
Chun, Y. S. et al. Significance of pathologic response to preoperative therapy in pancreatic cancer. Ann. Surg. Oncol. 18, 3601–3607 (2011).
Morton, D. et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial.J. Clin. Oncol. 41, 1541–1552 (2023).
Rugo, H. S. et al. Primary results from TROPiCS-02: a randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor-positive/HER2-negative (HR+/HER2-) advanced breast cancer. J. Clin. Oncol. 40, LBA1001–LBA1001 (2022).
Tagawa, S. T. et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J. Clin. Oncol. 39, 2474–2485 (2021).
Cortés, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Krop, I. E. et al. Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res. 18, 34 (2016).
Santin, A. D. et al. Safety and activity of anti-mesothelin antibody-drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian cancer: multicenter, phase Ib dose escalation and expansion study. Int. J. Gynecol. Cancer https://doi.org/10.1136/ijgc-2022-003927 (2022).
von Minkwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628 (2019).
Harbeck, N. et al. De-escalation strategies in human epidermal growth factor receptor 2 (HER2)–positive early breast cancer (BC): final analysis of the West German Study Group adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early BC HER2- and hormone receptor-positive phase II randomized trial — efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with or without endocrine therapy (ET) versus trastuzumab plus ET. J. Clin. Oncol. 35, 3046–3054 (2017).
Hoimes, C. J. et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J. Clin. Oncol. 41, 22–31 (2023).
Acknowledgements
No funding was provided for this work. We thank V. H. Goldstein of Dana-Farber Cancer Institute for submission assistance. The authors would also like to thank R. Colombo of Zymeworks (Vancouver, Canada) for providing precious insights during the writing of this paper. This article reflects the views of the authors and should not be construed to represent FDA views or policies.
Author information
Authors and Affiliations
Contributions
P.T. and B.R. researched data and wrote this manuscript. All authors made substantial contributions to discussions of content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.T. has acted as an adviser and/or consultant of AstraZeneca, Daiichi Sankyo, Gilead and Lilly. B.R. reports fees from Regeneron not related to the present article. S.M.T. has acted as a consultant and/or adviser of 4D Pharma, Aadi Bio, ARC Therapeutics, AstraZeneca, Bayer, BeyondSpring Pharmaceuticals, Blueprint Medicines, Bristol Myers Squibb, CytomX Therapeutics, Daiichi Sankyo, Eisai, Eli Lilly, Ellipses Pharma, Genentech/Roche, Gilead, Incyte Corporation, Infinity Therapeutics, Menarini/Stemline, Merck, Myovant, Novartis, Odonate Therapeutics, OncoSec Medical Inc., OncXerna, Pfizer, Reveal Genomics, Sanofi, Seattle Genetics, Umoja Biopharma, Zentalis, Zetagen, and Zymeworks and received research funding from AstraZeneca, Bristol Myers Squibb, Eisai, Exelixis, Gilead, Genentech/Roche, Lilly, Merck, NanoString Technologies, Novartis, OncoPep, Pfizer, Sanofi, and Seattle Genetics. S.M.P. declares no competing interests.
Peer review
Peer reviewer information
Nature Reviews Clinical Oncology thanks H. Burris III and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarantino, P., Ricciuti, B., Pradhan, S.M. et al. Optimizing the safety of antibody–drug conjugates for patients with solid tumours. Nat Rev Clin Oncol 20, 558–576 (2023). https://doi.org/10.1038/s41571-023-00783-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00783-w
This article is cited by
-
Exploring the next generation of antibody–drug conjugates
Nature Reviews Clinical Oncology (2024)
-
Pioneering the Way: The Revolutionary Potential of Antibody–Drug Conjugates in NSCLC
Current Treatment Options in Oncology (2024)