Abstract
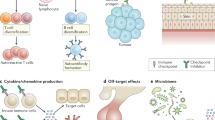
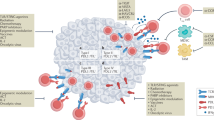
Many tumour antigens that do not arise from cancer cell-specific mutations are targets of humoral and cellular immunity despite their expression on non-malignant cells. Thus, in addition to the expected ability to detect mutations and stress-associated shifts in the immunoproteome and immunopeptidome (the sum of MHC class I-bound peptides) unique to malignant cells, the immune system also recognizes antigens expressed in non-malignant cells, which can result in autoimmune reactions against non-malignant cells from the tissue of origin. These autoimmune manifestations include, among others, vitiligo, thyroiditis and paraneoplastic syndromes, concurrent with melanoma, thyroid cancer and non-small-cell lung cancer, respectively. Importantly, despite the undesirable effects of these symptoms, such events can have prognostic value and correlate with favourable disease outcomes, suggesting ‘beneficial autoimmunity’. Similarly, the occurrence of dermal and endocrine autoimmune adverse events in patients receiving immune-checkpoint inhibitors can have a positive predictive value for therapeutic outcomes. Neoplasias derived from stem cells deemed ‘not essential’ for survival (such as melanocytes, thyroid cells and most cells in sex-specific organs) have a particularly good prognosis, perhaps because the host can tolerate autoimmune reactions that destroy tumour cells at some cost to non-malignant tissues. In this Perspective, we discuss examples of spontaneous as well as therapy-induced autoimmunity that correlate with favourable disease outcomes and make a strong case in favour of this ‘beneficial autoimmunity’ being important not only in patients with advanced-stage disease but also in cancer immunosurveillance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kroemer, G. & Martinez, C. The fail-safe paradigm of immunological self-tolerance. Lancet 338, 1246–1249 (1991).
Taniuchi, I. CD4 helper and CD8 cytotoxic T cell differentiation. Annu. Rev. Immunol. 36, 579–601 (2018).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018).
Kale, A., Sharma, A., Stolzing, A., Desprez, P. Y. & Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 17, 16 (2020).
Zindel, J. & Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 15, 493–518 (2020).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Gong, T., Liu, L., Jiang, W. & Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112 (2020).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Fridman, W. H., Zitvogel, L., Sautes-Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
Byrne, A. et al. Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nat. Rev. Clin. Oncol. 17, 341–348 (2020).
Shen, M., Wang, J. & Ren, X. New insights into tumor-infiltrating b lymphocytes in breast cancer: clinical impacts and regulatory mechanisms. Front. Immunol. 9, 470 (2018).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018).
Gros, A. et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 22, 433–438 (2016).
Stronen, E. et al. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 352, 1337–1341 (2016).
D’Alise, A. M. et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nat. Commun. 10, 2688 (2019).
Leko, V. & Rosenberg, S. A. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell 38, 454–472 (2020).
Dersh, D., Holly, J. & Yewdell, J. W. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat. Rev. Immunol. 21, 116–128 (2021).
von Kobbe, C. Targeting senescent cells: approaches, opportunities, challenges. Aging 11, 12844–12861 (2019).
Lopez-Otin, C. & Kroemer, G. Hallmarks of health. Cell 184, 33–63 (2021).
Gasser, S., Orsulic, S., Brown, E. J. & Raulet, D. H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 (2005).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Raulet, D. H. & Guerra, N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat. Rev. Immunol. 9, 568–580 (2009).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Palucka, A. K. & Coussens, L. M. The basis of oncoimmunology. Cell 164, 1233–1247 (2016).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Casares, N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202, 1691–1701 (2005).
Vacchelli, E. et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350, 972–978 (2015).
Galluzzi, L., Humeau, J., Buque, A., Zitvogel, L. & Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 17, 725–741 (2020).
Frank, M. J. et al. Autologous tumor cell vaccine induces antitumor T cell immune responses in patients with mantle cell lymphoma: A phase I/II trial. J. Exp. Med. 217, e20191712 (2020).
Lonial, S. et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 21, 207–221 (2020).
Kepp, O., Zitvogel, L. & Kroemer, G. Lurbinectedin: an FDA-approved inducer of immunogenic cell death for the treatment of small-cell lung cancer. Oncoimmunology 9, 1795995 (2020).
Buque, A. et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat. Commun. 11, 3819 (2020).
Roberti, M. P. et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat. Med. 26, 919–931 (2020).
Kooreman, N. G. et al. Autologous iPSC-based vaccines elicit anti-tumor responses in vivo. Cell Stem Cell 22, 501–513.e7 (2018).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020).
Semeraro, M. et al. The ratio of CD8+/FOXP3 T lymphocytes infiltrating breast tissues predicts the relapse of ductal carcinoma in situ. Oncoimmunology 5, e1218106 (2016).
Datta, J. et al. Progressive loss of anti-HER2 CD4+ T-helper type 1 response in breast tumorigenesis and the potential for immune restoration. Oncoimmunology 4, e1022301 (2015).
Nocera, N. F., Lee, M. C., De La Cruz, L. M., Rosemblit, C. & Czerniecki, B. J. Restoring lost anti-HER-2 Th1 immunity in breast cancer: a crucial role for Th1 cytokines in therapy and prevention. Front. Pharmacol. 7, 356 (2016).
Sharma, A. et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer 118, 4354–4362 (2012).
De La Cruz, L. M., Nocera, N. F. & Czerniecki, B. J. Restoring anti-oncodriver Th1 responses with dendritic cell vaccines in HER2/neu-positive breast cancer: progress and potential. Immunotherapy 8, 1219–1232 (2016).
Mascaux, C. et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature 571, 570–575 (2019).
Finn, O. J. The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 18, 183–194 (2018).
Jacqueline, C., Lee, A., Frey, N., Minden, J. S. & Finn, O. J. Inflammation-induced abnormal expression of self-molecules on epithelial cells: targets for tumor immunoprevention. Cancer Immunol. Res. 8, 1027–1038 (2020).
Jacqueline, C. & Finn, O. J. Antibodies specific for disease-associated antigens (DAA) expressed in non-malignant diseases reveal potential new tumor-associated antigens (TAA) for immunotherapy or immunoprevention. Semin. Immunol. 47, 101394 (2020).
Kvorjak, M. et al. Cross-talk between colon cells and macrophages increases ST6GALNAC1 and MUC1-sTn expression in ulcerative colitis and colitis-associated colon cancer. Cancer Immunol. Res. 8, 167–178 (2020).
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Gravett, A. M., Trautwein, N., Stevanovic, S., Dalgleish, A. G. & Copier, J. Gemcitabine alters the proteasome composition and immunopeptidome of tumour cells. Oncoimmunology 7, e1438107 (2018).
Oh, C. Y. et al. ALK and RET inhibitors promote HLA Class I antigen presentation and unmask new antigens within the tumor immunopeptidome. Cancer Immunol. Res. 7, 1984–1997 (2019).
Stopfer, L. E., Mesfin, J. M., Joughin, B. A., Lauffenburger, D. A. & White, F. M. Multiplexed relative and absolute quantitative immunopeptidomics reveals MHC I repertoire alterations induced by CDK4/6 inhibition. Nat. Commun. 11, 2760 (2020).
Galon, J. & Bruni, D. Tumor immunology and tumor evolution: intertwined histories. Immunity 52, 55–81 (2020).
Pennycuick, A. et al. Immune surveillance in clinical regression of preinvasive squamous cell lung cancer. Cancer Discov. 10, 1489–1499 (2020).
Giacomini, P., Fisher, P. B., Duigou, G. J., Gambari, R. & Natali, P. G. Regulation of class II MHC gene expression by interferons: insights into the mechanism of action of interferon (review). Anticancer. Res. 8, 1153–1161 (1988).
Muraro, E. et al. Fighting viral infections and virus-driven tumors with cytotoxic CD4+ T cells. Front. Immunol. 8, 197 (2017).
Oh, D. Y. et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 181, 1612–1625.e13 (2020).
Gencode Gene. Release 37 (GRCh38.p13). Gencode Gene https://www.gencodegenes.org/human (2020).
Ingolia, N. T. Ribosome profiling: new views of translation, from single codons to genome scale. Nat. Rev. Genet. 15, 205–213 (2014).
Chen, J. et al. Pervasive functional translation of noncanonical human open reading frames. Science 367, 1140–1146 (2020).
Brunet, M. A. et al. OpenProt: a more comprehensive guide to explore eukaryotic coding potential and proteomes. Nucleic Acids Res. 47, D403–D410 (2019).
Ouspenskaia, T. et al. Thousands of novel unannotated proteins expand the MHC I immunopeptidome in cancer. Preprint at bioRxiv https://doi.org/10.1101/2020.02.12.945840 (2020).
Larouche, J. D. et al. Widespread and tissue-specific expression of endogenous retroelements in human somatic tissues. Genome Med. 12, 40 (2020).
Erhard, F., Dolken, L., Schilling, B. & Schlosser, A. Identification of the cryptic HLA-I immunopeptidome. Cancer Immunol. Res. 8, 1018–1026 (2020).
Laumont, C. M. et al. Global proteogenomic analysis of human MHC class I-associated peptides derived from non-canonical reading frames. Nat. Commun. 7, 10238 (2016).
Laumont, C. M. et al. Non-coding regions are the main source of targetable tumor-specific antigens. Sci. Transl Med. 10, eaau5516 (2018).
Chong, C. et al. Integrated proteogenomic deep sequencing and analytics accurately identify non-canonical peptides in tumor immunopeptidomes. Nat. Commun. 11, 1293 (2020).
Berlin, C. et al. Mapping the HLA ligandome landscape of acute myeloid leukemia: a targeted approach toward peptide-based immunotherapy. Leukemia 29, 647–659 (2015).
Bilich, T. et al. The HLA ligandome landscape of chronic myeloid leukemia delineates novel T-cell epitopes for immunotherapy. Blood 133, 550–565 (2019).
Nelde, A. et al. HLA ligandome analysis of primary chronic lymphocytic leukemia (CLL) cells under lenalidomide treatment confirms the suitability of lenalidomide for combination with T-cell-based immunotherapy. Oncoimmunology 7, e1316438 (2018).
Shao, W. et al. The SysteMHC Atlas project. Nucleic Acids Res. 46, D1237–D1247 (2018).
Lanoix, J. et al. Comparison of the MHC I immunopeptidome repertoir of B-cell lymphoblasts using two isolation methods. Proteomics 18, e1700251 (2018).
Pfammatter, S. et al. Extending the comprehensiveness of immunopeptidome analyses using isobaric peptide labeling. Anal. Chem. 92, 9194–9204 (2020).
Boegel, S. et al. HLA and proteasome expression body map. BMC Med. Genomics 11, 36 (2018).
Benhammadi, M. et al. IFN lambda enhances constitutive expression of MHC class I molecules on thymic epithelial cells. J. Immunol. 205, 1268–1280 (2020).
Hukelmann, J. L. et al. The cytotoxic T cell proteome and its shaping by the kinase mTOR. Nat. Immunol. 17, 104–112 (2016).
Nagaraj, N. et al. Deep proteome and transcriptome mapping of a human cancer cell line. Mol. Syst. Biol. 7, 548 (2011).
Pearson, H. et al. MHC class I-associated peptides derive from selective regions of the human genome. J. Clin. Invest. 126, 4690–4701 (2016).
Muller, M., Gfeller, D., Coukos, G. & Bassani-Sternberg, M. ‘Hotspots’ of antigen presentation revealed by human leukocyte antigen ligandomics for neoantigen prioritization. Front. Immunol. 8, 1367 (2017).
Caron, E. et al. The MHC I immunopeptidome conveys to the cell surface an integrative view of cellular regulation. Mol. Syst. Biol. 7, 533 (2011).
Yewdell, J. W. & Holly, J. DRiPs get molecular. Curr. Opin. Immunol. 64, 130–136 (2020).
Demmers, L. C. et al. Single-cell derived tumor organoids display diversity in HLA class I peptide presentation. Nat. Commun. 11, 5338 (2020).
McGranahan, N. et al. Allele-specific HLA loss and immune escape in lung cancer evolution.Cell 171, 1259–1271.e11 (2017).
Garrido, F. MHC/HLA class I loss in cancer cells. Adv. Exp. Med. Biol. 1151, 15–78 (2019).
Dersh, D. et al. Genome-wide screens identify lineage- and tumor-specific genes modulating MHC-I- and MHC-II-restricted immunosurveillance of human lymphomas. Immunity 54, 116–131.e10 (2021).
Meunier, M. C. et al. T cells targeted against a single minor histocompatibility antigen can cure solid tumors. Nat. Med. 11, 1222–1229 (2005).
Kvistborg, P. et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology 1, 409–418 (2012).
Scheper, W. et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 25, 89–94 (2019).
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Coulie, P. G., van den Eynde, B. J., van der Bruggen, P. & Boon, T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer 14, 135–146 (2014).
Tran, E., Robbins, P. F. & Rosenberg, S. A. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 18, 255–262 (2017).
Apavaloaei, A., Hardy, M. P., Thibault, P. & Perreault, C. The origin and immune recognition of tumor-specific antigens. Cancers 12, 2607 (2020).
Löffler, M. W. et al. Multi-omics discovery of exome-derived neoantigens in hepatocellular carcinoma. Genome Med. 11, 1–16 (2019).
Van den Eynden, J., Jimenez-Sanchez, A., Miller, M. L. & Larsson, E. Lack of detectable neoantigen depletion signals in the untreated cancer genome. Nat. Genet. 51, 1741–1748 (2019).
Murata, K. et al. Landscape mapping of shared antigenic epitopes and their cognate TCRs of tumor-infiltrating T lymphocytes in melanoma. eLife 9, e53244 (2020).
Zhao, Q. et al. Proteogenomics uncovers a vast repertoire of shared tumor-specific antigens in ovarian cancer. Cancer Immunol. Res. 8, 544–555 (2020).
Bezu, L. et al. Trial watch: Peptide-based vaccines in anticancer therapy. Oncoimmunology 7, e1511506 (2018).
Haen, S. P., Loffler, M. W., Rammensee, H. G. & Brossart, P. Towards new horizons: characterization, classification and implications of the tumour antigenic repertoire. Nat. Rev. Clin. Oncol. 17, 595–610 (2020).
Weinzierl, A. O. et al. Distorted relation between mRNA copy number and corresponding major histocompatibility complex ligand density on the cell surface. Mol. Cell Proteom. 6, 102–113 (2007).
Kurts, C. et al. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc. Natl Acad. Sci. USA 96, 12703–12707 (1999).
Yu, W. et al. Clonal deletion prunes but does not eliminate self-specific alphabeta CD8+ T lymphocytes. Immunity 42, 929–941 (2015).
Richards, D. M., Kyewski, B. & Feuerer, M. Re-examining the nature and function of self-reactive T cells. Trends Immunol. 37, 114–125 (2016).
Delaney, J. R., Sykulev, Y., Eisen, H. N. & Tonegawa, S. Differences in the level of expression of class I major histocompatibility complex proteins on thymic epithelial and dendritic cells influence the decision of immature thymocytes between positive and negative selection. Proc. Natl Acad. Sci. USA 95, 5235–5240 (1998).
Malhotra, D. et al. Tolerance is established in polyclonal CD4+ T cells by distinct mechanisms, according to self-peptide expression patterns. Nat. Immunol. 17, 187–195 (2016).
Culina, S. et al. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci. Immunol. 3, eaao4013 (2018).
Klein, L., Kyewski, B., Allen, P. M. & Hogquist, K. A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol. 14, 377–391 (2014).
Cloosen, S. et al. Expression of tumor-associated differentiation antigens, MUC1 glycoforms and CEA, in human thymic epithelial cells: implications for self-tolerance and tumor therapy. Cancer Res. 67, 3919–3926 (2007).
Wells, D. K. et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 183, 818–834 (2020).
Krausgruber, T. et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020).
Esfahani, K. et al. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 17, 504–515 (2020).
Overwijk, W. W. et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4+ T lymphocytes. Proc. Natl Acad. Sci. USA 96, 2982–2987 (1999).
Steitz, J. et al. Genetic immunization of mice with human tyrosinase-related protein 2: implications for the immunotherapy of melanoma. Int. J. Cancer 86, 89–94 (2000).
Malik, B. T. et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol. 2, eaam6346 (2017).
Gonzalez Santiago, T. M., Wetter, D. A., Lowe, G. C. & Sciallis, G. F. Generalized discoid lupus erythematosus as the presenting sign of small cell lung carcinoma. Skinmed 15, 218–220 (2017).
Gonzalez Amores, Y., Hernando Rebollar, S. & Casado Bernabeu, A. Lupus as a paraneoplastic manifestation of cholangiocarcinoma. Rev. Esp. Enferm. Dig. 108, 292 (2016).
Maria, A. T. J. et al. Intriguing relationships between cancer and systemic sclerosis: role of the immune system and other contributors. Front. Immunol. 9, 3112 (2018).
Zerdes, I. et al. How can we effectively address the paraneoplastic dermatomyositis: diagnosis, risk factors and treatment options. J. BUON 22, 1073–1080 (2017).
Graus, F. & Dalmau, J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 16, 535–548 (2019).
Soomro, Z. et al. Paraneoplastic syndromes in small cell lung cancer. J. Thorac. Dis. 12, 6253–6263 (2020).
Przezdziecka-Dolyk, J. et al. Ocular paraneoplastic syndromes. Biomedicines 8, 490 (2020).
Cao, L. et al. Chronic lymphocytic leukemia-associated paraneoplastic pemphigus: potential cause and therapeutic strategies. Sci. Rep. 10, 16357 (2020).
Okamoto, A., Watanabe, T., Kamata, K., Minaga, K. & Kudo, M. Recent updates on the relationship between cancer and autoimmune pancreatitis. Intern. Med. 58, 1533–1539 (2019).
Sugiyama, T. et al. A case of high-grade pancreatic intraepithelial neoplasia concomitant with type 1 autoimmune pancreatitis: The process underlying both conditions. Pathol. Int. 69, 165–171 (2019).
Albert, M. L. et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat. Med. 4, 1321–1324 (1998).
Yshii, L. M., Hohlfeld, R. & Liblau, R. S. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat. Rev. Neurol. 13, 755–763 (2017).
Vogrig, A. et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 6, e604 (2019).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Evans, R. L., Pottala, J. V., Nagata, S. & Egland, K. A. Longitudinal autoantibody responses against tumor-associated antigens decrease in breast cancer patients according to treatment modality. BMC Cancer 18, 119 (2018).
Wang, S. et al. Using a panel of multiple tumor-associated antigens to enhance autoantibody detection for immunodiagnosis of gastric cancer. Oncoimmunology 7, e1452582 (2018).
Koziol, J. A., Imai, H., Dai, L., Zhang, J. Y. & Tan, E. M. Early detection of hepatocellular carcinoma using autoantibody profiles from a panel of tumor-associated antigens. Cancer Immunol. Immunother. 67, 835–841 (2018).
Li, P. et al. Evaluation of serum autoantibodies against tumor-associated antigens as biomarkers in lung cancer. Tumour Biol. 39, 1010428317711662 (2017).
Michels, J. et al. Multiplex bead-based measurement of humoral immune responses against tumor-associated antigens in stage II melanoma patients of the EORTC18961 trial. Oncoimmunology 7, e1428157 (2018).
Sun, H. et al. Serum autoantibodies against a panel of 15 tumor-associated antigens in the detection of ovarian cancer. Tumour Biol. 39, 1010428317699132 (2017).
Dieu-Nosjean, M. C. et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26, 4410–4417 (2008).
Sautes-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Maoz, A., Dennis, M. & Greenson, J. K. The Crohn’s-like lymphoid reaction to colorectal cancer-tertiary lymphoid structures with immunologic and potentially therapeutic relevance in colorectal cancer. Front. Immunol. 10, 1884 (2019).
Myshunina, T. M., Guda, B. D., Bolgov, M. Y., Mikhailenko, N. I. & Tronko, N. D. Differentiated thyroid carcinomas associated with chronic thyroiditis: biological and clinical properties. Exp. Oncol. 40, 128–131 (2018).
Moon, S. et al. Associations between Hashimoto thyroiditis and clinical outcomes of papillary thyroid cancer: a meta-analysis of observational studies. Endocrinol. Metab. 33, 473–484 (2018).
Gozzard, P., Chapman, C., Vincent, A., Lang, B. & Maddison, P. Novel humoral prognostic markers in small-cell lung carcinoma: a prospective study. PLoS ONE 10, e0143558 (2015).
Maddison, P., Gozzard, P., Grainge, M. J. & Lang, B. Long-term survival in paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 88, 1334–1339 (2017).
Motofei, I. G. Melanoma and autoimmunity: spontaneous regressions as a possible model for new therapeutic approaches. Melanoma Res. 29, 231–236 (2019).
Paradisi, A. et al. Markedly reduced incidence of melanoma and nonmelanoma skin cancer in a nonconcurrent cohort of 10,040 patients with vitiligo. J. Am. Acad. Dermatol. 71, 1110–1116 (2014).
Bae, J. M. et al. Markedly reduced risk of internal malignancies in patients with vitiligo: a nationwide population-based cohort study. J. Clin. Oncol. 37, 903–911 (2019).
Teulings, H. E. et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J. Clin. Oncol. 33, 773–781 (2015).
Zhou, X. et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 18, 87 (2020).
De Angelis, R. et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol. 15, 23–34 (2014).
Montesion, M. et al. Somatic HLA class I loss is a widespread mechanism of immune evasion which refines the use of tumor mutational burden as a biomarker of checkpoint inhibitor response. Cancer Discov. 11, 282–292 (2021).
Nakamura, Y. et al. Nivolumab therapy for treatment-related vitiligo in a patient with relapsed metastatic melanoma. JAMA Dermatol. 153, 942–944 (2017).
Nardin, C., Pelletier, F., Puzenat, E. & Aubin, F. Vitiligo repigmentation with melanoma progression during pembrolizumab treatment. Acta Derm. Venereol. 99, 913–914 (2019).
Babai, S., Voisin, A. L., Bertin, C., Gouverneur, A. & Le-Louet, H. Occurrences and outcomes of immune checkpoint inhibitors-induced vitiligo in cancer patients: a retrospective cohort study. Drug. Saf. 43, 111–117 (2020).
Suresh, K. et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J. Thorac. Oncol. 14, 494–502 (2019).
Fukihara, J. et al. Prognostic Impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin. Lung Cancer 20, 442–450.e4 (2019).
Lei, M., Michael, A., Patel, S. & Wang, D. Evaluation of the impact of thyroiditis development in patients receiving immunotherapy with programmed cell death-1 inhibitors. J. Oncol. Pharm. Pract. 25, 1402–1411 (2019).
Patel, V. et al. Acute interstitial nephritis, a potential predictor of response to immune checkpoint inhibitors in renal cell carcinoma. J. Immunother. Cancer 8, e001198 (2020).
Bulik-Sullivan, B. et al. Deep learning using tumor HLA peptide mass spectrometry datasets improves neoantigen identification. Nat. Biotechnol. 37, 55–63 (2019).
Qian, S. B. et al. Tight linkage between translation and MHC class I peptide ligand generation implies specialized antigen processing for defective ribosomal products. J. Immunol. 177, 227–233 (2006).
Abelin, J. G. et al. Mass spectrometry profiling of HLA-associated peptidomes in mono-allelic cells enables more accurate epitope prediction. Immunity 46, 315–326 (2017).
Martins, R. P. et al. Nuclear processing of nascent transcripts determines synthesis of full-length proteins and antigenic peptides. Nucleic Acids Res. 47, 3086–3100 (2019).
Apcher, S. et al. Translation of pre-spliced RNAs in the nuclear compartment generates peptides for the MHC class I pathway. Proc. Natl Acad. Sci. USA 110, 17951–17956 (2013).
Wei, J. et al. Ribosomal proteins regulate MHC class I peptide generation for immunosurveillance. Mol. Cell 73, 1162–1173.e5 (2019).
Yewdell, J. W., Dersh, D. & Fahraeus, R. Peptide channeling: the key to MHC class I immunosurveillance? Trends Cell Biol. 29, 929–939 (2019).
Wei, J. et al. Varied role of ubiquitylation in generating MHC class i peptide ligands. J. Immunol. 198, 3835–3845 (2017).
Bassani-Sternberg, M., Pletscher-Frankild, S., Jensen, L. J. & Mann, M. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol. Cell Proteom. 14, 658–673 (2015).
Forlani, G. et al. CIITA-transduced glioblastoma cells uncover a rich repertoire of clinically relevant tumor-associated HLA-II antigens. Mol. Cell Proteomics 20, 100032 (2020).
de Verteuil, D. et al. Deletion of immunoproteasome subunits imprints on the transcriptome and has a broad impact on peptides presented by major histocompatibility complex I molecules. Mol. Cell Proteom. 9, 2034–2047 (2010).
Vigneron, N. & Van den Eynde, B. J. Proteasome subtypes and the processing of tumor antigens: increasing antigenic diversity. Curr. Opin. Immunol. 24, 84–91 (2012).
Granados, D. P. et al. ER stress affects processing of MHC class I-associated peptides. BMC Immunol. 10, 10 (2009).
Wahl, A., Schafer, F., Bardet, W. & Hildebrand, W. H. HLA class I molecules reflect an altered host proteome after influenza virus infection. Hum. Immunol. 71, 14–22 (2010).
Jaeger, A. M. et al. Rebalancing protein homeostasis enhances tumor antigen presentation. Clin. Cancer Res. 25, 6392–6405 (2019).
Bloy, N. et al. Immunogenic stress and death of cancer cells: contribution of antigenicity vs adjuvanticity to immunosurveillance. Immunol. Rev. 280, 165–174 (2017).
Rozek, L. S. et al. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J. Natl Cancer Inst. 108, djw027 (2016).
Quaglino, P. et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann. Oncol. 21, 409–414 (2010).
Turajlic, S. et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 18, 1009–1021 (2017).
Liu, P. et al. Immunosuppression by mutated calreticulin released from malignant cells. Mol. Cell 77, 748–760.e9 (2020).
Laumont, C. M. et al. Noncoding regions are the main source of targetable tumor-specific antigens. Sci. Transl Med. 10, eaau5516 (2018).
Acknowledgements
We thank V. Carbonnier (Centre de Recherche des Cordeliers, Paris, France) for help in preparing Fig. 2. L.Z. and G.K. receive support from the Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Ruban Rose”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085); Inserm (HTE); Institut National du Cancer (INCa); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); Ligue contre le Cancer (équipe labellisée); RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. C.P. receives support from the Canadian Cancer Society (#705604) and the Canadian Institutes of Health Research (FDN 148400). O.J.F. receives support from the University of Pittsburgh Medical Center (UPMC) Immune Transplant and Therapy Center (ITTC) and the US National Cancer Institute (grant 5R35CA210039).
Author information
Authors and Affiliations
Contributions
G.K. researched data for the manuscript, L.Z. and G.K. wrote it with the input of C.P. and O.J.F., and all authors edited and reviewed the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
L.Z. and G.K. are founders of EverImmune. G.K. is a founder of Samsara Therapeutics and Therafast Bio. O.J.F. is a consultant for Biovelocita, GeoVax, Immodulon, Iaso Therapeutics and PDS Biotech. C.P. declares no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks S. Karagiannis, R. Liblau, H-G. Rammensee and J. Yewdell for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Antigen spreading
-
Expansion of the immune response to secondary epitopes that were not part of the initial recognition process.
- Danger-associate molecular patterns
-
(DAMPs). Protein exposed on the surface of stressed cells or metabolite or protein released from stressed or dying cells that alerts immune effectors.
- Disease-associated antigens
-
Self-molecules that undergo changes in levels of expression and post-translational modifications in cells infected with pathogens, subjected to acute inflammation or during malignant transformation.
- Immunopeptidome
-
Sum of peptides bound to MHC class I molecules that can be recognized by T cell receptors.
- Immune-related adverse events
-
(irAEs). Undesired adverse events of immune-checkpoint inhibitors caused by excessive activation of autoimmune responses.
- Lambert–Eaton myasthenic syndrome
-
Myasthenia-like condition in which an autoimmune response to the neuromuscular junctions causes progressive muscle weakness. Half of the diagnoses occur in patients with small-cell lung cancer.
- Medullary thymic epithelial cells
-
(mTECs). Specialized cells that present self-antigens to induce clonal deletion of self-specific T lymphocytes, thus assuring central tolerance.
- Melanocytes
-
Specialized cells found in the basal skin layer that produce melanin and transfer this pigment in the form of melanosomes into the cytoplasm of adjacent keratinocytes.
- Paraneoplastic syndromes
-
Compendium of clinical signs provoked by a cancer at a distant location, generally as a result of autoimmune responses.
- Senescence
-
Close-to-irreversible blockade of the cell cycle coupled to a major loss of cell function that occurs in response to injury or as a result of aging.
- Thyrocyte
-
Thyroid epithelial cell producing thyroid hormones.
- Tumour-associated antigens
-
Antigens that can be found on non-malignant cells but whose (over)expression on the surface of cancer cells enables the immune system to distinguish such cells from their non-transformed counterparts.
- Tumour-specific antigens
-
Antigens that are uniquely present within or on a cancer cell. They can arise via both mutational (mutated tumour-specific antigens) and non-mutational (non-mutated tumour-specific antigens) mechanisms.
- Vitiligo
-
Also known as leukoderma. White patches of the skin caused by the autoimmune destruction of melanocytes.
Rights and permissions
About this article
Cite this article
Zitvogel, L., Perreault, C., Finn, O.J. et al. Beneficial autoimmunity improves cancer prognosis. Nat Rev Clin Oncol 18, 591–602 (2021). https://doi.org/10.1038/s41571-021-00508-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00508-x
This article is cited by
-
Elucidating the role of Pyroptosis in papillary thyroid cancer: prognostic, immunological, and therapeutic perspectives
Cancer Cell International (2024)
-
Immunogenic cell death in cancer: concept and therapeutic implications
Journal of Translational Medicine (2023)
-
Bodywide ecological interventions on cancer
Nature Medicine (2023)
-
Proteomics to study cancer immunity and improve treatment
Seminars in Immunopathology (2023)
-
Identification of antigenic epitopes recognized by tumor infiltrating lymphocytes in high grade serous ovarian cancer by multi-omics profiling of the auto-antigen repertoire
Cancer Immunology, Immunotherapy (2023)