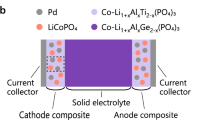
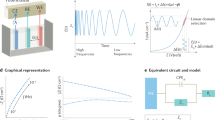
Abstract
The functions of electrochemical energy conversion and storage devices rely on the dynamic junction between a solid and a fluid: the electrochemical interface (EI). Many experimental techniques have been developed to probe the EI, but they provide only a partial picture. Building a full mechanistic understanding requires combining multiple probes, either successively or simultaneously. However, such combinations lead to important technical and theoretical challenges. In this Review, we focus on complementary optoelectronic probes and modelling to address the EI across different timescales and spatial scales — including mapping surface reconstruction, reactants and reaction modulators during operation. We discuss how combining these probes can facilitate a predictive design of the EI when closely integrated with theory.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Tian, Y. et al. Promises and challenges of next-generation ‘beyond Li-ion’ batteries for electric vehicles and grid decarbonization. Chem. Rev. 121, 1623–1669 (2021).
Frith, J. T., Lacey, M. J. & Ulissi, U. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 14, 420 (2023).
Pastor, E. et al. Spectroelectrochemical analysis of the mechanism of (photo)electrochemical hydrogen evolution at a catalytic interface. Nat. Commun. 8, 14280 (2017).
Gratzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001).
Sivula, K. & van de. Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 1, 15010 (2016).
Sivula, K. Toward economically feasible direct solar-to-fuel energy conversion. J. Phys. Chem. Lett. 6, 975–976 (2015).
Sullivan, I. et al. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 4, 952–958 (2021).
Stephens, I. E. L. et al. 2022 roadmap on low temperature electrochemical CO2 reduction. J. Phys. Energy 4, 042003 (2022).
Wang, M. & Feng, Z. Interfacial processes in electrochemical energy systems. Chem. Commun. 57, 10453–10468 (2021).
Schmickler, W. Electrochemical theory: double layer. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering (Elsevier, 2014).
Shin, S.-J. et al. On the importance of the electric double layer structure in aqueous electrocatalysis. Nat. Commun. 13, 174 (2022).
Sebastián-Pascual, P., Shao-Horn, Y. & Escudero-Escribano, M. Toward understanding the role of the electric double layer structure and electrolyte effects on well-defined interfaces for electrocatalysis. Curr. Opin. Electrochem. 32, 100918 (2022).
Adler, S.B. et al. In High-Temperature Solid Oxide Fuel Cells for the 21st Century (eds Kendall K. & Kendall M.) 2nd edn, ix–x (Academic Press, 2016).
Glenk, G. & Reichelstein, S. Reversible power-to-gas systems for energy conversion and storage. Nat. Commun. 13, 2010 (2022).
Ye, L., Duan, X. & Xie, K. Electrochemical oxidative dehydrogenation of ethane to ethylene in a solid oxide electrolyzer. Angew. Chem. Int. Ed. 60, 21746–21750 (2021).
Schmickler, W. Electronic effects in the electric double layer. Chem. Rev. 96, 3177–3200 (1996).
Bard, A. J., Faulkner, L. R. & White, H. L. In Electrochemical Methods: Fundamentals and Applications 3rd edn (Wiley, 2022).
Pastor, E. et al. The role of crystal facets and disorder on photo-electrosynthesis. Nanoscale 14, 15596–15606 (2022).
Hong, W. T. et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Monteiro, M. C. O., Dattila, F., López, N. & Koper, M. T. M. The role of cation acidity on the competition between hydrogen evolution and CO2 reduction on gold electrodes. J. Am. Chem. Soc. 144, 1589–1602 (2022).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Yang, Y. et al. Operando methods: a new era of electrochemistry. Curr. Opin. Electrochem. 42, 101403 (2023).
Maibach, J., Rizell, J., Matic, A. & Mozhzhukhina, N. Toward operando characterization of interphases in batteries. ACS Mater. Lett. 5, 2431–2444 (2023).
Gourdin, G. & Doan-Nguyen, V. In situ, operando characterization of materials for electrochemical devices. Cell Rep. Phys. Sci. 2, 100660 (2021).
Zaera, F. Probing liquid/solid interfaces at the molecular level. Chem. Rev. 112, 2920–2986 (2012).
Cheng, W., Su, H. & Liu, Q. Tracking the oxygen dynamics of solid–liquid electrochemical interfaces by correlative in situ synchrotron spectroscopies. Acc. Chem. Res. 55, 1949–1959 (2022).
Deng, J. et al. Understanding photoelectrochemical water oxidation with X-ray absorption spectroscopy. ACS Energy Lett. 5, 975–993 (2020).
Wang, X., Huang, S.-C., Hu, S., Yan, S. & Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2, 253–271 (2020).
Wang, S. et al. Electrochemical impedance spectroscopy. Nat. Rev. Methods Prim. 1, 41 (2021).
Du, J. et al. In situ crystallization of active NiOOH/CoOOH heterostructures with hydroxide ion adsorption sites on velutipes-like CoSe/NiSe nanorods as catalysts for oxygen evolution and cocatalysts for methanol oxidation. ACS Appl. Mater. Interf. 12, 686–697 (2020).
Polo-Garzon, F., Bao, Z., Zhang, X., Huang, W. & Wu, Z. Surface reconstructions of metal oxides and the consequences on catalytic chemistry. ACS Catal. 9, 5692–5707 (2019).
Liu, X. et al. Comprehensive understandings into complete reconstruction of precatalysts: synthesis, applications, and characterizations. Adv. Mater. 33, 2007344 (2021).
Sarma, B. B., Maurer, F., Doronkin, D. E. & Grunwaldt, J.-D. Design of single-atom catalysts and tracking their fate using operando and advanced X-ray spectroscopic tools. Chem. Rev. 123, 379–444 (2023).
Lassalle-Kaiser, B., Gul, S., Kern, J., Yachandra, V. K. & Yano, J. In situ/operando studies of electrocatalysts using hard X-ray spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 221, 18–27 (2017).
Timoshenko, J. & Cuenya, B. R. In situ/operando electrocatalyst characterization by X-ray absorption spectroscopy. Chem. Rev. 121, 882–961 (2021).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2020).
Vavra, J. et al. Solution-based Cu+ transient species mediate the reconstruction of copper electrocatalysts for CO2 reduction. Nat. Catal. 7, 89–97 (2024).
Grumelli, D. et al. Electrochemical stability of the reconstructed Fe3O4(001) surface. Angew. Chem. Int. Ed. 59, 21904–21908 (2020).
Zhu, C. et al. Product-specific active site motifs of Cu for electrochemical CO2 reduction. Chem 7, 406–420 (2021).
Ahn, S. T., Sen, S. & Palmore, G. T. R. Grazing incidence X-ray diffraction: identifying the dominant facet in copper foams that electrocatalyze the reduction of carbon dioxide to formate. Nanoscale 14, 13132–13140 (2022).
Hejral, U., Shipilin, M., Gustafson, J., Stierle, A. & Lundgren, E. High energy surface X-ray diffraction applied to model catalyst surfaces at work. J. Phys. Condens. Matter 33, 073001 (2020).
Zu, L. et al. Self-assembly of Ir-based nanosheets with ordered interlayer space for enhanced electrocatalytic water oxidation. J. Am. Chem. Soc. 144, 2208–2217 (2022).
Wu, X.-L. et al. Self-templated synthesis of novel carbon nanoarchitectures for efficient electrocatalysis. Sci. Rep. 6, 28049 (2016).
Nyman, M. Small-angle X-ray scattering to determine solution speciation of metal-oxo clusters. Coord. Chem. Rev. 352, 461–472 (2017).
Yu, W., Fu, H. J., Mueller, T., Brunschwig, B. S. & Lewis, N. S. Atomic force microscopy: emerging illuminated and operando techniques for solar fuel research. J. Chem. Phys. 153, 020902 (2020).
Simon, G. H., Kley, C. S. & Roldan Cuenya, B. Potential-dependent morphology of copper catalysts during CO2 electroreduction revealed by in situ atomic force microscopy. Angew. Chem. Int. Ed. 60, 2561–2568 (2021).
Munz, M., Poon, J., Frandsen, W., Roldan Cuenya, B. & Kley, C. S. Nanoscale electron transfer variations at electrocatalyst–electrolyte interfaces resolved by in situ conductive atomic force microscopy. J. Am. Chem. Soc. 145, 5242–5251 (2023).
Zhu, X., Revilla, R. I. & Hubin, A. Direct correlation between local surface potential measured by Kelvin probe force microscope and electrochemical potential of LiNi0.80Co0.15Al0.05O2 cathode at different state of charge. J. Phys. Chem. C 122, 28556–28563 (2018).
Li, S., Zhou, Y., Zi, Y., Zhang, G. & Wang, Z. L. Excluding contact electrification in surface potential measurement using Kelvin probe force microscopy. ACS Nano 10, 2528–2535 (2016).
Nesbitt, N. T. & Smith, W. A. Operando topography and mechanical property mapping of CO2 reduction gas-diffusion electrodes operating at high current densities. J. Electrochem. Soc. 168, 044505 (2021).
Wang, Z., Ke, X. & Sui, M. Recent progress on revealing 3D structure of electrocatalysts using advanced 3D electron tomography: a mini review. Front. Chem. 10, 872117 (2022).
Meyer, Q., Zeng, Y. & Zhao, C. In situ and operando characterization of proton exchange membrane fuel cells. Adv. Mater. 31, 1901900 (2019).
Matsui, H. et al. Operando 3D visualization of migration and degradation of a platinum cathode catalyst in a polymer electrolyte fuel cell. Angew. Chem. Int. Ed. 129, 9499–9503 (2017).
Seitzman, N. et al. Operando X-ray tomography imaging of solid-state electrolyte response to Li evolution under realistic operating conditions. ACS Appl. Energy Mater. 4, 1346–1355 (2021).
Vamvakeros, A. et al. Real-time tomographic diffraction imaging of catalytic membrane reactors for the oxidative coupling of methane. Catal. Today 364, 242–255 (2021).
Vamvakeros, A. et al. 5D operando tomographic diffraction imaging of a catalyst bed. Nat. Commun. 9, 4751 (2018).
Tang, P. & Arbiol, J. Engineering surface states of hematite based photoanodes for boosting photoelectrochemical water splitting. Nanoscale Horiz. 4, 1256–1276 (2019).
Zhang, X. et al. From rational design of a new bimetallic MOF family with tunable linkers to OER catalysts. J. Mater. Chem. A 7, 1616–1628 (2019).
Zhang, X. et al. Tailor-made metal–nitrogen–carbon bifunctional electrocatalysts for rechargeable Zn–air batteries via controllable MOF units. Energy Storage Mater. 17, 46–61 (2019).
He, Y. et al. Engineering grain boundaries at the 2D limit for the hydrogen evolution reaction. Nat. Commun. 11, 57 (2020).
He, Y. et al. Amorphizing noble metal chalcogenide catalysts at the single-layer limit towards hydrogen production. Nat. Catal. 5, 212–221 (2022).
Liang, Z. et al. Molecular engineering to tune the ligand environment of atomically dispersed nickel for efficient alcohol electrochemical oxidation. Adv. Funct. Mater. 31, 2106349 (2021).
Zhang, T. et al. Site-specific axial oxygen coordinated FeN4 active sites for highly selective electroreduction of carbon dioxide. Adv. Funct. Mater. 32, 2111446 (2022).
Yang, D. et al. A high conductivity 1D π–d conjugated metal–organic framework with efficient polysulfide trapping-diffusion-catalysis in lithium–sulfur batteries. Adv. Mater. 34, 2108835 (2022).
Liang, Z. et al. A novel π–d conjugated cobalt tetraaza[14]annulene based atomically dispersed electrocatalyst for efficient CO2 reduction. Chem. Eng. J. 442, 136129 (2022).
Liang, Z. et al. Molecular engineering to introduce carbonyl between nickel salophen active sites to enhance electrochemical CO2 reduction to methanol. Appl. Catal. B 314, 121451 (2022).
Han, X. et al. Engineering the interfacial microenvironment via surface hydroxylation to realize the global optimization of electrochemical CO2 reduction. ACS Appl. Mater. Interf. 14, 32157–32165 (2022).
Zhang, T. et al. Quasi-double-star nickel and iron active sites for high-efficiency carbon dioxide electroreduction. Energy Environ. Sci. 14, 4847–4857 (2021).
Han, X., Zhang, T. & Arbiol, J. Metal–organic framework-derived single atom catalysts for electrocatalytic reduction of carbon dioxide to C1 products. Energy Adv. 2, 252–267 (2023).
Tang, P.-Y. et al. Boosting photoelectrochemical water oxidation of hematite in acidic electrolytes by surface state modification. Adv. Energy Mater. 9, 1901836 (2019).
Tang, P. et al. Enhanced photoelectrochemical water splitting of hematite multilayer nanowire photoanodes by tuning the surface state via bottom-up interfacial engineering. Energy Environ. Sci. 10, 2124–2136 (2017).
Hansen, P. L. et al. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 295, 2053–2055 (2002).
Hodnik, N., Dehm, G. & Mayrhofer, K. J. J. Importance and challenges of electrochemical in situ liquid cell electron microscopy for energy conversion research. Acc. Chem. Res. 49, 2015–2022 (2016).
Ruiz-Zepeda, F. et al. Atomically resolved anisotropic electrochemical shaping of nano-electrocatalyst. Nano Lett. 19, 4919–4927 (2019).
Altantzis, T. et al. Three-dimensional quantification of the facet evolution of Pt nanoparticles in a variable gaseous environment. Nano Lett. 19, 477–481 (2019).
van Omme, J. T. et al. Liquid phase transmission electron microscopy with flow and temperature control. J. Mater. Chem. C 8, 10781–10790 (2020).
Beker, A. F. et al. In situ electrochemistry inside a TEM with controlled mass transport. Nanoscale 12, 22192–22201 (2020).
Huang, Y. et al. Unraveling dynamical behaviors of zinc metal electrodes in aqueous electrolytes through an operando study. Energy Storage Mater. 46, 243–251 (2022).
Yang, Y. et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023).
Botifoll, M., Pinto-Huguet, I. & Arbiol, J. Machine learning in electron microscopy for advanced nanocharacterization: current developments, available tools and future outlook. Nanoscale Horiz. 7, 1427–1477 (2022).
Wang, H.-L., You, E.-M., Panneerselvam, R., Ding, S.-Y. & Tian, Z.-Q. Advances of surface-enhanced Raman and IR spectroscopies: from nano/microstructures to macro-optical design. Light. Sci. Appl. 10, 161 (2021).
Zhu, S., Li, T., Cai, W.-B. & Shao, M. CO2 electrochemical reduction as probed through infrared spectroscopy. ACS Energy Lett. 4, 682–689 (2019).
Papasizza, M. & Cuesta, A. In situ monitoring using ATR-SEIRAS of the electrocatalytic reduction of CO2 on Au in an ionic liquid/water mixture. ACS Catal. 8, 6345–6352 (2018).
Yang, K., Kas, R. & Smith, W. A. In situ infrared spectroscopy reveals persistent alkalinity near electrode surfaces during CO2 electroreduction. J. Am. Chem. Soc. 141, 15891–15900 (2019).
Han, X. X., Rodriguez, R. S., Haynes, C. L., Ozaki, Y. & Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Prim. 1, 87 (2022).
Devasia, D., Wilson, A. J., Heo, J., Mohan, V. & Jain, P. K. A rich catalog of C–C bonded species formed in CO2 reduction on a plasmonic photocatalyst. Nat. Commun. 12, 2612 (2021).
An, H. et al. Sub-second time-resolved surface-enhanced Raman spectroscopy reveals dynamic CO intermediates during electrochemical CO2 reduction on copper. Angew. Chem. Int. Ed. 60, 16576–16584 (2021).
Galloway, T. A. & Hardwick, L. J. Utilizing in situ electrochemical SHINERS for oxygen reduction reaction studies in aprotic electrolytes. J. Phys. Chem. Lett. 7, 2119–2124 (2016).
Ojha, K., Arulmozhi, N., Aranzales, D. & Koper, M. T. M. Double layer at the Pt(111)–aqueous electrolyte interface: potential of zero charge and anomalous Gouy–Chapman screening. Angew. Chem. Int. Ed. 59, 711–715 (2020).
Ojha, K., Doblhoff-Dier, K. & Koper, M. T. M. Double-layer structure of the Pt(111)–aqueous electrolyte interface. Proc. Natl Acad. Sci. USA 119, e2116016119 (2022).
Doyle, R. L. & Lyons, M. E. G. An electrochemical impedance study of the oxygen evolution reaction at hydrous iron oxide in base. Phys. Chem. Chem. Phys. 15, 5224–5237 (2013).
Danaee, I., Jafarian, M., Forouzandeh, F., Gobal, F. & Mahjani, M. G. Electrochemical impedance studies of methanol oxidation on GC/Ni and GC/NiCu electrode. Int. J. Hydrog. Energy 34, 859–869 (2009).
Connor, P., Schuch, J., Kaiser, B. & Jaegermann, W. The determination of electrochemical active surface area and specific capacity revisited for the system MnOx as an oxygen evolution catalyst. Z. Phys. Chem. 234, 979–994 (2020).
McCrory, C. C. L., Jung, S., Peters, J. C. & Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977–16987 (2013).
Hegner, F. S. et al. Cobalt hexacyanoferrate on BiVO4 photoanodes for robust water splitting. ACS Appl. Mater. Interf. 9, 37671–37681 (2017).
Noguera-Gómez, J. et al. Solution-processed Ni-based nanocomposite electrocatalysts: an approach to highly efficient electrochemical water splitting. ACS Appl. Energy Mater. 4, 5255–5264 (2021).
Corby, S. et al. Separating bulk and surface processes in NiOx electrocatalysts for water oxidation. Sustain. Energy Fuels 4, 5024–5030 (2020).
Gimenez, S. et al. Carrier density and interfacial kinetics of mesoporous TiO2 in aqueous electrolyte determined by impedance spectroscopy. J. Electroanal. Chem. 668, 119–125 (2012).
Bisquert, J., Giménez, S., Bertoluzzi, L. & Herraiz-Cardona, I. Analysis of photoelectrochemical systems by impedance spectroscopy. In Photoelectrochemical Solar Fuel Production: From Basic Principles to Advanced Devices (eds Giménez, S. & Bisquert, J.) 281–321 (Springer, 2016).
Heijne, A. T. et al. Identifying charge and mass transfer resistances of an oxygen reducing biocathode. Energy Environ. Sci. 4, 5035–5043 (2011).
Ledezma-Yanez, I. et al. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2, 17031 (2017).
Lefebvre, M. C., Martin, R. B. & Pickup, P. G. Characterization of ionic conductivity profiles within proton exchange membrane fuel cell gas diffusion electrodes by impedance spectroscopy. Electrochem. Solid-State Lett. 2, 259 (1999).
Favaro, M. et al. Unravelling the electrochemical double layer by direct probing of the solid/liquid interface. Nat. Commun. 7, 12695 (2016).
Xu, P., von Rueden, A. D., Schimmenti, R., Mavrikakis, M. & Suntivich, J. Optical method for quantifying the potential of zero charge at the platinum–water electrochemical interface. Nat. Mater. 22, 503–510 (2023).
Zheng, W. Beginner’s guide to Raman spectroelectrochemistry for electrocatalysis study. Chem. Methods 3, e202200042 (2023).
Mozhzhukhina, N. et al. Direct operando observation of double layer charging and early solid electrolyte interphase formation in Li-ion battery electrolytes. J. Phys. Chem. Lett. 11, 4119–4123 (2020).
Wang, Y.-H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021).
Lee, J. et al. Investigating the effects of gas diffusion layer substrate thickness on polymer electrolyte membrane fuel cell performance via synchrotron X-ray radiography. Electrochim. Acta 236, 161–170 (2017).
Bui, J. C., Kim, C., Weber, A. Z. & Bell, A. T. Dynamic boundary layer simulation of pulsed CO2 electrolysis on a copper catalyst. ACS Energy Lett. 6, 1181–1188 (2021).
Timoshenko, J. et al. Steering the structure and selectivity of CO2 electroreduction catalysts by potential pulses. Nat. Catal. 5, 259–267 (2022).
Lee, S. H. et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ X-ray characterization. J. Am. Chem. Soc. 143, 588–592 (2021).
García de Arquer, F. P. et al. 2D metal oxyhalide-derived catalysts for efficient CO2 electroreduction. Adv. Mater. 30, 1802858 (2018).
He, S. et al. The p-orbital delocalization of main-group metals to boost CO2 electroreduction. Angew. Chem. Int. Ed. 57, 16114–16119 (2018).
Lei, Q. et al. Structural evolution and strain generation of derived-Cu catalysts during CO2 electroreduction. Nat. Commun. 13, 4857 (2022).
Vavra, J., Shen, T.-H., Stoian, D., Tileli, V. & Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem. Int. Ed. 60, 1347–1354 (2021).
Grosse, P. et al. Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nat. Commun. 12, 6736 (2021).
Jeon, H. S. et al. Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction. J. Am. Chem. Soc. 141, 19879–19887 (2019).
Pardo Pérez, L. C. et al. Determining structure-activity relationships in oxide derived Cu–Sn catalysts during CO2 electroreduction using X-ray spectroscopy. Adv. Energy Mater. 12, 2103328 (2022).
Burdyny, T. & Smith, W. A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 12, 1442–1453 (2019).
Velasco-Velez, J.-J. et al. Revealing the active phase of copper during the electroreduction of CO2 in aqueous electrolyte by correlating in situ X-ray spectroscopy and in situ electron microscopy. ACS Energy Lett. 5, 2106–2111 (2020).
Pastor, E. et al. Electronic defects in metal oxide photocatalysts. Nat. Rev. Mater. 7, 503–521 (2022).
Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016).
Divins, N. J., Angurell, I., Escudero, C., Pérez-Dieste, V. & Llorca, J. Influence of the support on surface rearrangements of bimetallic nanoparticles in real catalysts. Science 346, 620–623 (2014).
Jovanovič, P. et al. Electrochemical dissolution of iridium and iridium oxide particles in acidic media: transmission electron microscopy, electrochemical flow cell coupled to inductively coupled plasma mass spectrometry, and X-ray absorption spectroscopy study. J. Am. Chem. Soc. 139, 12837–12846 (2017).
Yoo, M. et al. A tailored oxide interface creates dense Pt single-atom catalysts with high catalytic activity. Energy Environ. Sci. 13, 1231–1239 (2020).
Wu, Y. A. et al. Facet-dependent active sites of a single Cu2O particle photocatalyst for CO2 reduction to methanol. Nat. Energy 4, 957–968 (2019).
Zhang, R. et al. Compositionally complex doping for zero-strain zero-cobalt layered cathodes. Nature 610, 67–73 (2022).
Li, Y. et al. Complex structural dynamics of nanocatalysts revealed in operando conditions by correlated imaging and spectroscopy probes. Nat. Commun. 6, 7583 (2015).
Parker, J. E. et al. A cell design for correlative hard X-ray nanoprobe and electron microscopy studies of catalysts under in situ conditions. J. Synchrotron Radiat. 29, 431–438 (2022).
Chen, C., Hayazawa, N. & Kawata, S. A 1.7 nm resolution chemical analysis of carbon nanotubes by tip-enhanced Raman imaging in the ambient. Nat. Commun. 5, 3312 (2014).
Pienpinijtham, P., Kitahama, Y. & Ozaki, Y. Progress of tip-enhanced Raman scattering for the last two decades and its challenges in very recent years. Nanoscale 14, 5265–5288 (2022).
Stöckle, R. M., Suh, Y. D., Deckert, V. & Zenobi, R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem. Phys. Lett. 318, 131–136 (2000).
Nguyen, D. et al. Probing molecular-scale catalytic interactions between oxygen and cobalt phthalocyanine using tip-enhanced raman spectroscopy. J. Am. Chem. Soc. 140, 5948–5954 (2018).
Zeng, Z.-C. et al. Electrochemical tip-enhanced raman spectroscopy. J. Am. Chem. Soc. 137, 11928–11931 (2015).
Altman, E. I., Baykara, M. Z. & Schwarz, U. D. Noncontact atomic force microscopy: an emerging tool for fundamental catalysis research. Acc. Chem. Res. 48, 2640–2648 (2015).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Albrecht, F. et al. Selectivity in single-molecule reactions by tip-induced redox chemistry. Science 377, 298–301 (2022).
Wagner, M., Meyer, B., Setvin, M., Schmid, M. & Diebold, U. Direct assessment of the acidity of individual surface hydroxyls. Nature 592, 722–725 (2021).
Mefford, J. T. et al. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature 593, 67–73 (2021).
Selim, S. et al. Impact of oxygen vacancy occupancy on charge carrier dynamics in BiVO4 photoanodes. J. Am. Chem. Soc. 141, 18791–18798 (2019).
Arcas, R. et al. Direct observation of the chemical transformations in BiVO4 photoanodes upon prolonged light‐aging treatments. Sol. RRL 6, 2200132 (2022).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Beverskog, B. & Puigdomenech, I. Revised Pourbaix diagrams for iron at 25–300 °C. Corros. Sci. 38, 2121–2135 (1996).
Hansen, H. A., Rossmeisl, J. & Nørskov, J. K. Surface Pourbaix diagrams and oxygen reduction activity of Pt, Ag and Ni(111) surfaces studied by DFT. Phys. Chem. Chem. Phys. 10, 3722–3730 (2008).
Dattila, F., Garcı́a-Muelas, R. & López, N. Active and selective ensembles in oxide-derived copper catalysts for CO2 reduction. ACS Energy Lett. 5, 3176–3184 (2020).
Ren, D. et al. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper(I) oxide catalysts. ACS Catal. 5, 2814–2821 (2015).
Ugeda, M. M. et al. Giant bandgap renormalization and excitonic effects in a monolayer transition metal dichalcogenide semiconductor. Nat. Mater. 13, 1091–1095 (2014).
Feenstra, R. M. & Stroscio, J. A. Tunneling spectroscopy of the GaAs(110) surface. J. Vac. Sci. Technol. B 5, 923–929 (1987).
Barja, S. et al. Identifying substitutional oxygen as a prolific point defect in monolayer transition metal dichalcogenides. Nat. Commun. 10, 3382 (2019).
Salmeron, M. & Eren, B. High-pressure scanning tunneling microscopy. Chem. Rev. 121, 962–1006 (2021).
Zhang, H. et al. Recent progress with in situ characterization of interfacial structures under a solid–gas atmosphere by HP-STM and AP-XPS. Materials 12, 3674 (2019).
Böller, B., Durner, K. M. & Wintterlin, J. The active sites of a working Fischer–Tropsch catalyst revealed by operando scanning tunnelling microscopy. Nat. Catal. 2, 1027–1034 (2019).
Favaro, M. et al. Elucidating the alkaline oxygen evolution reaction mechanism on platinum. J. Mater. Chem. A 5, 11634–11643 (2017).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Zheng, Y. et al. High electrocatalytic hydrogen evolution activity of an anomalous ruthenium catalyst. J. Am. Chem. Soc. 138, 16174–16181 (2016).
Zhou, Y. et al. Long-chain hydrocarbons by CO2 electroreduction using polarized nickel catalysts. Nat. Catal. 5, 545–554 (2022).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Hörmann, N. G., Andreussi, O. & Marzari, N. Grand canonical simulations of electrochemical interfaces in implicit solvation models. J. Chem. Phys. 150, 041730 (2019).
Sundararaman, R., Goddard, W. A. III & Arias, T. A. Grand canonical electronic density-functional theory: algorithms and applications to electrochemistry. J. Chem. Phys. 146, 114104 (2017).
Zhan, C. et al. Revealing the CO coverage-driven C–C coupling mechanism for electrochemical CO2 reduction on Cu2O nanocubes via operando Raman spectroscopy. ACS Catal. 11, 7694–7701 (2021).
Velasco-Velez, J. J. et al. Photoelectron spectroscopy at the graphene–liquid interface reveals the electronic structure of an electrodeposited cobalt/graphene electrocatalyst. Angew. Chem. Int. Ed. 54, 14554–14558 (2015).
Casalongue, H. S. et al. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode. Nat. Commun. 4, 2817 (2013).
Saveleva, V. A. et al. Uncovering the stabilization mechanism in bimetallic ruthenium–iridium anodes for proton exchange membrane electrolyzers. J. Phys. Chem. Lett. 7, 3240–3245 (2016).
Casalongue, H. G. S. et al. Operando characterization of an amorphous molybdenum sulfide nanoparticle catalyst during the hydrogen evolution reaction. J. Phys. Chem. C 118, 29252–29259 (2014).
Favaro, M. et al. An operando investigation of (Ni–Fe–Co–Ce)Ox system as highly efficient electrocatalyst for oxygen evolution reaction. ACS Catal. 7, 1248–1258 (2017).
Zenyuk, I. V. et al. Investigating evaporation in gas diffusion layers for fuel cells with X-ray computed tomography. J. Phys. Chem. C 120, 28701–28711 (2016).
Haussener, S., Suter, S. & Gutierrez Perez, R. Solar fuels devices: multi-scale modeling and device design guidelines. In Springer Handbook of Inorganic Photochemistry (eds Bahnemann, D. & Patrocinio, A. O. T.) 965–983 (Springer, 2022).
Wakerley, D. et al. Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers. Nat. Energy 7, 130–143 (2022).
Hacene, M. et al. Accelerating VASP electronic structure calculations using graphic processing units. J. Comput. Chem. 33, 2581–2589 (2012).
Glaser, J. et al. Strong scaling of general-purpose molecular dynamics simulations on GPUs. Comput. Phys. Commun. 192, 97–107 (2015).
Chang, C., Deringer, V. L., Katti, K. S., Van Speybroeck, V. & Wolverton, C. M. Simulations in the era of exascale computing. Nat. Rev. Mater. 8, 309–313 (2023).
Gavini, V. et al. Roadmap on electronic structure codes in the exascale era. Model. Simul. Mater. Sci. Eng. 31, 063301 (2023).
Manathunga, M., Aktulga, H. M., Götz, A. W. & Merz, K. M. Jr Quantum mechanics/molecular mechanics simulations on NVIDIA and AMD graphics processing units. J. Chem. Inf. Model. 63, 711–717 (2023).
Yu, V. W. & Govoni, M. GPU acceleration of large-scale full-frequency GW calculations. J. Chem. Theory Comput. 18, 4690–4707 (2022).
Wang, Z., Guo, X., Montoya, J. & Nørskov, J. K. Predicting aqueous stability of solid with computed Pourbaix diagram using SCAN functional. npj Comput. Mater. 6, 160 (2020).
Bracco G. & Holst B. In Surface Science Techniques (Springer, 2013).
Bergmann, U. & Glatzel, P. X-ray emission spectroscopy. Photosynth. Res. 102, 255–266 (2009).
Corby, S. et al. Charge separation, band-bending, and recombination in WO 3 photoanodes. J. Phys. Chem. Lett. 10, 5395–5401 (2019).
Pastor, E. et al. Nonthermal breaking of magnetic order via photogenerated spin defects in the spin-orbit coupled insulator Sr 3 Ir 2 O 7. Phys. Rev. B 105, 064409 (2022).
Mesa, C. A. et al. Multihole water oxidation catalysis on haematite photoanodes revealed by operando spectroelectrochemistry and DFT. Nat. Chem. 12, 82–89 (2020).
Mesa, C. A., Pastor, E. & Francàs, L. UV–vis operando spectroelectrochemistry for (photo)electrocatalysis: principles and guidelines. Curr. Opin. Electrochem. 35, 101098 (2022).
Billinge, S. J. L. The rise of the X-ray atomic pair distribution function method: a series of fortunate events. Phil. Trans. R. Soc. Math. Phys. Eng. Sci. 377, 20180413 (2019).
Cliffe, M. J., Dove, M. T., Drabold, D. A. & Goodwin, A. L. Structure determination of disordered materials from diffraction data. Phys. Rev. Lett. 104, 125501 (2010).
Williams, D. B. & Carter, C. B. Transmission Electron Microscopy (Springer, 2009).
Davies, P. R. & Morgan, D. J. Practical guide for X-ray photoelectron spectroscopy: applications to the study of catalysts. J. Vac. Sci. Technol. A 38, 033204 (2020).
Hansen, W. N. & Kolb, D. M. The work function of emersed electrodes. J. Electroanal. Chem. Interfacial Electrochem. 100, 493–500 (1979).
Stuve, E. M., Krasnopoler, A. & Sauer, D. E. Relating the in-situ, ex-situ, and non-situ environments in surface electrochemistry. Surf. Sci. 335, 177–185 (1995).
Reniers, F. The development of a transfer mechanism between UHV and electrochemistry environments. J. Phys. Appl. Phys. 35, R169 (2002).
Soriaga, M. P. et al. Electrochemical surface science of CO2 reduction at well-defined Cu electrodes: surface characterization by emersion, ex situ, in situ, and operando methods. In Encyclopedia of Interfacial Chemistry (ed. Wandelt, K.) 562–576 (Elsevier, 2018).
Woehl, T. J., Moser, T., Evans, J. E. & Ross, F. M. Electron-beam-driven chemical processes during liquid phase transmission electron microscopy. MRS Bull. 45, 746–753 (2020).
Lian, Z., Yang, M., Jan, F. & Li, B. Machine learning derived blueprint for rational design of the effective single-atom cathode catalyst of the lithium–sulfur battery. J. Phys. Chem. Lett. 12, 7053–7059 (2021).
Pablo-García, S. et al. Mechanistic routes toward C3 products in copper-catalysed CO2 electroreduction. Catal. Sci. Technol. 12, 409–417 (2022).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Acknowledgements
The authors thank the following projects for support: RED2022-134508-T, PID2021-124796OB-I00, PID2020-116093RB-C41, PID2020-116093RB-C43 and PID2020-116093RB-C44, funded by MCIN/AEI. The Institut de Ciències Fotòniques (ICFO) acknowledges CEX2019-000910-S, Fundació Cellex, Fundació Mir-Puig and the La Caixa Foundation (100010434, EU Horizon 2020 Marie Skłodowska-Curie grant agreement 847648). The Institute of Chemical Research of Catalonia (ICIQ) acknowledges support from the Ministerio de Ciencia e Innovación through the ‘Severo Ochoa’ Excellence Accreditation CEX2021-001214-S. D.E. acknowledges funding from PID2019-108532GB-I00, PID2022-136961NB-I00, QUIMTRONIC-CM Y2018/NMT-4783, the European Union NextGenerationEU (PRTR-C17.I1) (MAD2D-CM)-MRR project, and ERC CoG 766555. IMDEA Nanoscience acknowledges support from the ‘Severo Ochoa’ Program for Centers of Excellence in R&D (CEX2020-001039-S). The Catalan Institute of Nanoscience and Nanotechnology (ICN2) acknowledges support from MCIN/AEI for CEX2021-001214-S, NextGenerationEU (PRTR-C17.I1) PID2020-116093RB-C41 and PID2020-116093RB-C43, and the Generalitat de Catalunya for 2021SGR00457 and PRTR-C17.I1. S.B. acknowledges support from the grants RYC-2017-21931 and EUR2020-112066 funded by MCIN/AEI, from ESF Investing in your future, from the European Union NextGenerationEU/PRTR, and from the Basque Government (grant IT1591-22). ICFO, ICIQ and ICN2 are funded by CERCA — Generalitat de Catalunya. Z.L. and N.L. acknowledge support from Marie Skłodowska-Curie grant agreement number 101064867, the Spanish Ministry of Science and Innovation (PID2021-122516OB-I00 and the ‘Severo Ochoa’ Program for Centers of Excellence CEX2019-000925-S). E.P. acknowledges the support of the CNRS and the French Agence Nationale de la Recherche (ANR), under grant ANR-22-CPJ2-0053-01.
Author information
Authors and Affiliations
Contributions
E.P. and F.P.G.d.A. conceived the initial idea and led article preparation. All authors contributed to the writing and editing of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CSIC-Crystallography: https://www.xtal.iqfr.csic.es/Cristalografia/index-en.html
The Electrochemical Society, the birth of electrochemistry: https://www.electrochem.org/birth-of-electrochemistry
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pastor, E., Lian, Z., Xia, L. et al. Complementary probes for the electrochemical interface. Nat Rev Chem 8, 159–178 (2024). https://doi.org/10.1038/s41570-024-00575-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-024-00575-5
This article is cited by
-
Electrochemical hydrogenation and oxidation of organic species involving water
Nature Reviews Chemistry (2024)