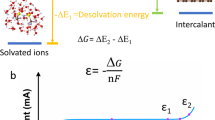
Abstract
Anions serve as an essential component of electrolytes, whose effects have long been ignored. However, since the 2010s, we have seen a considerable increase of anion chemistry research in a range of energy storage devices, and it is now understood that anions can be well tuned to effectively improve the electrochemical performance of such devices in many aspects. In this Review, we discuss the roles of anion chemistry across various energy storage devices and clarify the correlations between anion properties and their performance indexes. We highlight the effects of anions on surface and interface chemistry, mass transfer kinetics and solvation sheath structure. Finally, we conclude with a perspective on the challenges and opportunities of anion chemistry for enhancing specific capacity, output voltage, cycling stability and anti-self-discharge ability of energy storage devices.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yu, X. et al. Emergent pseudocapacitance of 2D nanomaterials. Adv. Energy Mater. 8, 1702930 (2018).
Gund, G. S. et al. MXene/polymer hybrid materials for flexible AC-filtering electrochemical capacitors. Joule 3, 164–176 (2019).
Placke, T. et al. Perspective on performance, cost, and technical challenges for practical dual-ion batteries. Joule 2, 2528–2550 (2018).
Tan, J., Matz, J., Dong, P., Ye, M. & Shen, J. Appreciating the role of polysulfides in lithium-sulfur batteries and regulation strategies by electrolytes engineering. Energy Storage Mater. 42, 645–678 (2021).
Dou, Q. et al. Emerging trends in anion storage materials for the capacitive and hybrid energy storage and beyond. Chem. Soc. Rev. 50, 6734–6789 (2021).
Osaka, T., Naoi, K. & Ogano, S. Effect of polymerization anion on electrochemical properties of polypyrrole and on Li/LiClO4/polypyrrole battery performance. J. Electrochem. Soc. 135, 1071–1077 (1988).
McCullough, F.P., Levine, C.A. & Snelgrove, R.V. Secondary battery. US Patent 4830938 (1989).
Carlin, R. T. et al. Dual intercalating molten electrolyte batteries. Mater. Res. Soc. Symp. Proc. 393, 201–206 (1995).
Chaumont, A. & Wipff, G. Halide anion solvation and recognition by a macrotricyclic tetraammonium host in an ionic liquid: a molecular dynamics study. New J. Chem. 30, 537–545 (2006).
Lota, G. & Frackowiak, E. Electrochemistry communications striking capacitance of carbon/iodide interface. Electrochem. Commun. 11, 87–90 (2009).
Reddy, M. A. & Fichtner, M. Batteries based on fluoride shuttle. J. Mater. Chem. 21, 17059–17062 (2011).
Burke, C. M. et al. Enhancing electrochemical intermediate solvation through electrolyte anion selection to increase nonaqueous Li-O2 battery capacity. Proc. Natl Acad. Sci. USA 112, 9293–9298 (2015).
Liu, Z., Cui, T., Lu, T., Shapouri Ghazvini, M. & Endres, F. Anion effects on the solid/ionic liquid interface and the electrodeposition of zinc. J. Phys. Chem. C 120, 20224–20231 (2016).
Smaran, K. S. et al. Anion-dependent potential precycling effects on lithium deposition/dissolution reaction studied by an electrochemical quartz crystal microbalance. J. Phys. Chem. Lett. 8, 5203–5208 (2017).
Zhao, C. Z. et al. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc. Natl Acad. Sci. USA 114, 11069–11074 (2017). This work shows that the anions in polymer electrolyte have important effects on transfer number of their charge carriers and mass transfer kinetics.
Chao, D. & Fan, H. J. Intercalation pseudocapacitive behavior powers aqueous batteries. Chem 5, 1359–1361 (2019).
Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 86, 119–126 (2012).
Choi, C. et al. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 5, 5–19 (2020).
Aldama, I. et al. Contribution of cations and anions of aqueous electrolytes to the charge stored at the electric electrolyte/electrode interface of carbon-based supercapacitors. J. Phys. Chem. C 121, 12053–12062 (2017).
Suleman, M., Kumar, Y. & Hashmi, S. A. Solid-state electric double layer capacitors fabricated with plastic crystal based flexible gel polymer electrolytes: effective role of electrolyte anions. Mater. Chem. Phys. 163, 161–171 (2015).
Kato, K., Rodrigues, M. T. F., Babu, G. & Ajayan, P. M. Revealing anion chemistry above 3V in Li-ion capacitors. Electrochim. Acta 324, 134871 (2019).
Huang, Z. et al. Manipulating anion intercalation enables a high-voltage aqueous dual ion battery. Nat. Commun. 12, 3106 (2021). This work demonstrates that the output voltage of dual-ion batteries can be manipulated by the solvation energy of anions.
Li, J. et al. Anion and cation substitution in transition-metal oxides nanosheets for high-performance hybrid supercapacitors. Nano Energy 57, 22–33 (2019).
Sivakumar, P. et al. Ultrafast and reversible anion storage of spinel nanoarchitecture for high-performance alkaline zinc full cells. Appl. Phys. Rev. 8, 021408 (2021).
Shao, H., Wu, Y. C., Lin, Z., Taberna, P. L. & Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 49, 3005–3039 (2020).
Jin, H. et al. Heteroatom-doped porous carbon materials with unprecedented high volumetric capacitive performance. Angew. Chem. Int. Ed. 131, 2419–2423 (2019).
Feng, D. et al. Robust and conductive two-dimensional metal–organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 3, 30–36 (2018).
Li, B. et al. Electrolytic-anion-redox adsorption pseudocapacitance in nanosized lithium-free transition metal oxides as cathode materials for Li-ion batteries. Nano Energy 72, 104727 (2020).
Boisset, A. et al. Comparative performances of birnessite and cryptomelane MnO2 as electrode material in neutral aqueous lithium salt for supercapacitor application. J. Phys. Chem. C 117, 7408–7422 (2013).
Mefford, J. T. et al. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 13, 726–732 (2014).
Liu, Y. et al. Design of perovskite oxides as anion-intercalation-type electrodes for supercapacitors: cation leaching effect. ACS Appl. Mater. Interfaces 8, 23774–23783 (2016).
Han, C. et al. Boost anion storage capacity using conductive polymer as a pseudocapacitive cathode for high-energy and flexible lithium ion capacitors. ACS Appl. Mater. Interfaces 12, 10479–10489 (2020).
Huang, Z. et al. Effects of anion carriers on capacitance and self-discharge behaviors of zinc ion capacitors. Angew. Chem. Int. Ed. 60, 1011–1021 (2021). This work proposes that the anti-self-discharge ability of supercapacitor is determined by the stability of adsorbed structure of anions and cathode materials.
Qin, W. et al. Mini-review on the redox additives in aqueous electrolyte for high performance supercapacitors. ACS Omega 5, 3801–3808 (2020).
Sun, K. et al. A simple and high-performance supercapacitor based on nitrogen-doped porous carbon in redox-mediated sodium molybdate electrolyte. Electrochim. Acta 158, 361–367 (2015).
Jayaramulu, K. et al. Ultrathin hierarchical porous carbon nanosheets for high-performance supercapacitors and redox electrolyte energy storage. Adv. Mater. 30, 1705789 (2018).
Roldán, S. et al. Towards a further generation of high-energy carbon-based capacitors by using redox-active electrolytes. Angew. Chem. Int. Ed. 50, 1699–1701 (2011).
Skunik-Nuckowska, M., Węgrzyn, K., Dyjak, S., Wisińska, N. H. & Kulesza, P. J. Polyoxometalate/hydroquinone dual redox electrolyte for hybrid energy storage systems. Energy Storage Mater. 21, 427–438 (2019).
Mourad, E. et al. Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat. Mater. 16, 446–454 (2017). This work demonstrates that tethering redox groups to ionic liquids can greatly increase the specific energy and maintain the fast redox kinetics of supercapacitor.
Schmuelling, G. et al. X-ray diffraction studies of the electrochemical intercalation of bis(trifluoromethanesulfonyl)imide anions into graphite for dual-ion cells. J. Power Sources 239, 563–571 (2013).
Wang, Y. et al. Ultrafast charging and stable cycling dual-ion batteries enabled via an artificial cathode–electrolyte interface. Adv. Funct. Mater. 31, 2102360 (2021).
Xiang, L. et al. Highly concentrated electrolyte towards enhanced energy density and cycling life of dual-ion battery. Angew. Chem. Int. Ed. 59, 17924–17930 (2020).
Xu, X. et al. Quasi-solid-state dual-ion sodium metal batteries for low-cost energy storage. Chem 6, 902–918 (2020).
Yang, K. et al. Locally ordered graphitized carbon cathodes for high‐capacity dual‐ion batteries. Angew. Chem. Int. Ed. 133, 6396–6402 (2021).
Wang, M. et al. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 10, 667–672 (2018).
Kravchyk, K. V., Seno, C. & Kovalenko, M. V. Limitations of chloroaluminate ionic liquid anolytes for aluminum-graphite dual-ion batteries. ACS Energy Lett. 5, 545–549 (2020).
Wang, G. et al. A high-voltage, dendrite-free, and durable Zn–graphite battery. Adv. Mater. 32, 1905681 (2020).
Zhang, X., Tang, Y., Zhang, F. & Lee, C. S. A novel aluminum–graphite dual-ion battery. Adv. Energy Mater. 6, 1502588 (2016).
Tong, X. et al. Carbon-coated porous aluminum foil anode for high-rate, long-term cycling stability, and high energy density dual-ion batteries. Adv. Mater. 28, 9979–9985 (2016).
Li, C. et al. Preparation of Si-graphite dual-ion batteries by tailoring the voltage window of pretreated Si-anodes. Mater. Today Energy 8, 174–181 (2018).
Jiang, C. et al. Flexible interface design for stress regulation of a silicon anode toward highly stable dual-ion batteries. Adv. Mater. 32, 1908470 (2020).
Yu, M. et al. Interlayer gap widened α-phase molybdenum trioxide as high-rate anodes for dual-ion-intercalation energy storage devices. Nat. Commun. 11, 1348 (2020).
Sheng, M., Zhang, F., Ji, B., Tong, X. & Tang, Y. A novel tin-graphite dual-ion battery based on sodium-ion electrolyte with high energy density. Adv. Energy Mater. 7, 1601963 (2017).
Ji, B., Zhang, F., Song, X. & Tang, Y. A novel potassium-ion-based dual-ion battery. Adv. Mater. 29, 1700519 (2017).
Gao, J., Yoshio, M., Qi, L. & Wang, H. Solvation effect on intercalation behaviour of tetrafluoroborate into graphite electrode. J. Power Sources 278, 452–457 (2015).
Kravchyk, K. V. et al. High-energy-density dual-ion battery for stationary storage of electricity using concentrated potassium fluorosulfonylimide. Nat. Commun. 9, 4469 (2018).
Chen, Z. et al. Anion solvation reconfiguration enables high-voltage carbonate electrolytes for stable Zn/graphite cells. Angew. Chem. Int. Ed. 59, 21769–21777 (2020).
Chen, Z. et al. Fast anion intercalation into graphite cathode enabling high-rate rechargeable zinc batteries. J. Power Sources 457, 227994 (2020).
Meister, P. et al. Dual-ion cells based on the electrochemical intercalation of asymmetric fluorosulfonyl-(trifluoromethanesulfonyl) imide anions into graphite. Electrochim. Acta 130, 625–633 (2014).
Wang, D. Y. et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 8, 14283 (2017).
Wang, S. et al. A novel dual-graphite aluminum-ion battery. Energy Storage Mater. 12, 119–127 (2018).
Wrogemann, J. M. et al. Development of safe and sustainable dual-ion batteries through hybrid aqueous/nonaqueous electrolytes. Adv. Energy Mater. 10, 1902709 (2020).
Zhu, J. et al. Hybrid aqueous/nonaqueous water-in-bisalt electrolyte enables safe dual ion batteries. Small 16, 1905838 (2020).
Kim, K. et al. [LiCl2]− superhalide: a new charge carrier for graphite cathode of dual-ion batteries. Adv. Funct. Mater. 32, 2112709 (2022).
Zhang, H., Liu, X., Qin, B. & Passerini, S. Electrochemical intercalation of anions in graphite for high-voltage aqueous zinc battery. J. Power Sources 449, 227594 (2020).
Guo, Q. et al. A high-potential anion-insertion carbon cathode for aqueous zinc dual-ion battery. Adv. Funct. Mater. 30, 2002825 (2020).
Aubrey, M. L. & Long, J. R. A dual-ion battery cathode via oxidative insertion of anions in a metal–organic framework. J. Am. Chem. Soc. 137, 13594–13602 (2015).
Rodríguez-Pérez, I. A. et al. A hydrocarbon cathode for dual-ion batteries. ACS Energy Lett. 1, 719–723 (2016).
Fan, L., Liu, Q., Xu, Z. & Lu, B. An organic cathode for potassium dual-ion full battery. ACS Energy Lett. 2, 1614–1620 (2017).
Walter, M., Kravchyk, K. V., Böfer, C., Widmer, R. & Kovalenko, M. V. Polypyrenes as high-performance cathode materials for aluminum batteries. Adv. Mater. 30, 1705644 (2018).
Huang, H. et al. Design and construction of few-layer graphene cathode for ultrafast and high-capacity aluminum-ion batteries. Energy Storage Mater. 27, 396–404 (2020).
Li, C. et al. Dual-ion battery with MoS2 cathode. Energy Storage Mater. 32, 159–166 (2020).
Chen, F. et al. A dual-ion electrochemistry deionization system based on AgCl-Na0.44MnO2 electrodes. Nanoscale 9, 10101–10108 (2017).
Zhao, W. et al. Ultrahigh-desalination-capacity dual-ion electrochemical deionization device based on Na3V2(PO4)3@C-AgCl electrodes. ACS Appl. Mater. Interfaces 10, 40540–40548 (2018).
Pasta, M. et al. A desalination battery. Nano Lett. 12, 839–843 (2012).
Zhao, W. et al. Dual-ion electrochemical deionization system with binder-free aerogel electrodes. Small 15, 1805505 (2019).
Chen, F. et al. Dual-ions electrochemical deionization: a desalination generator. Energy Environ. Sci. 10, 2081–2089 (2017).
Kong, H. et al. Polypyrrole as a novel chloride-storage electrode for seawater desalination. Energy Technol. 7, 1900835 (2019).
Wu, X. et al. Reverse dual-ion battery via a ZnCl2 water-in-salt electrolyte. J. Am. Chem. Soc. 141, 6338–6344 (2019).
Wang, G., Yu, M. & Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 50, 2388–2443 (2021).
Kravchyk, K. V. & Kovalenko, M. V. Rechargeable dual-ion batteries with graphite as a cathode: key challenges and opportunities. Adv. Energy Mater. 9, 1901749 (2019).
Rodríguez-Pérez, I. A. & Ji, X. Anion hosting cathodes in dual-ion batteries. ACS Energy Lett. 2, 1762–1770 (2017).
Ji, X. A paradigm of storage batteries. Energy Environ. Sci. 12, 3203–3224 (2019).
Ji, B., Yao, W. & Tang, Y. High-performance rechargeable zinc-based dual-ion batteries. Sustain. Energy Fuels 4, 101–107 (2019).
Rodríguez-Pérez, I. A. et al. Enabling natural graphite in high-voltage aqueous graphite || Zn metal dual-ion batteries. Adv. Energy Mater. 10, 2001256 (2020).
Zhang, L. et al. Large-sized few-layer graphene enables an ultrafast and long-life aluminum-ion battery. Adv. Energy Mater. 7, 1700034 (2017).
Ko, S., Yamada, Y. & Yamada, A. An overlooked issue for high-voltage Li-ion batteries: suppressing the intercalation of anions into conductive carbon. Joule 5, 998–1009 (2021).
Li, Q. et al. Both cationic and anionic Co-(de)intercalation into a metal-oxide material. Joule 2, 1134–1145 (2018). This work demonstrates that co-(de)intercalation of anions and cations could reach a capacity beyond the theoretical capacity for conventional metal oxides with only cationic (de)intercalation.
Wang, S. et al. Regulating fast anionic redox for high-voltage aqueous hydrogen-ion-based energy storage. Angew. Chem. Int. Ed. 58, 205–210 (2019).
Zeng, X. et al. Toward a reversible Mn4+/Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2 batteries via salt anion chemistry. Adv. Energy Mater. 10, 1904163 (2020).
Bitenc, J. et al. Effect of Cl− and TFSI− anions on dual electrolyte systems in a hybrid Mg/Li4Ti5O12 battery. Electrochem. Commun. 76, 29–33 (2017).
Tezel, A. O. et al. Effect of a boron based anion receptor on graphite and LiFePO4 electrodes. J. Electrochem. Soc. 167, 020525 (2020).
Xu, H. et al. Strong anion receptor-assisted boron-based Mg electrolyte with wide electrochemical window and non-nucleophilic characteristic. Electrochem. Commun. 83, 72–76 (2017).
Li, Y. et al. Hexagonal boron nitride induces anion trapping in a polyethylene oxide based solid polymer electrolyte for lithium dendrite inhibition. J. Mater. Chem. A 8, 9579–9589 (2020).
Lutz, L. et al. Role of electrolyte anions in the Na-O2 battery: implications for NaO2 solvation and the stability of the sodium solid electrolyte interphase in Glyme ethers. Chem. Mater. 29, 6066–6075 (2017).
Sankarasubramanian, S., Kahky, J. & Ramani, V. Tuning anion solvation energetics enhances potassium–oxygen battery performance. Proc. Natl Acad. Sci. USA 116, 14899–14904 (2019).
Leverick, G. et al. Solvent- and anion-dependent Li+-O2-coupling strength and implications on the thermodynamics and kinetics of Li-O2 batteries. J. Phys. Chem. C 124, 4953–4967 (2020).
Chu, H. et al. Achieving three-dimensional lithium sulfide growth in lithium–sulfur batteries using high-donor-number anions. Nat. Commun. 10, 188 (2019).
Chu, H. et al. Unraveling the dual functionality of high-donor-number anion in lean-electrolyte lithium–sulfur batteries. Adv. Energy Mater. 10, 2000493 (2020).
Yang, B. et al. Critical role of anion donicity in Li2S deposition and sulfur utilization in Li-S batteries. ACS Appl. Mater. Interfaces 11, 25940–25948 (2019).
Han, K. S. et al. Effects of anion mobility on electrochemical behaviors of lithium–sulfur batteries. Chem. Mater. 29, 9023–9029 (2017).
Cao, R. et al. Effect of the anion activity on the stability of Li metal anodes in lithium–sulfur batteries. Adv. Funct. Mater. 26, 3059–3066 (2016).
Nowroozi, M. A. et al. Fluoride ion batteries — past, present, and future. J. Mater. Chem. A 9, 5980–6012 (2021).
Okazaki, K. I. et al. Charge–discharge behavior of bismuth in a liquid electrolyte for rechargeable batteries based on a fluoride shuttle. ACS Energy Lett. 2, 1460–1464 (2017).
Davis, V. K. et al. Room-temperature cycling of metal fluoride electrodes: liquid electrolytes for high-energy fluoride ion cells. Science 362, 1144–1148 (2018).
Li, X. et al. Initiating a room-temperature rechargeable aqueous fluoride-ion battery with long lifespan through a rational buffering phase design. Adv. Energy Mater. 11, 2003714 (2021).
Konishi, H. et al. Triphenylboroxine and triphenylborane as anion acceptors for electrolyte in fluoride shuttle batteries. Chem. Lett. 47, 1346–1349 (2018).
Konishi, H. et al. Effect of anion acceptor added to the electrolyte on the electrochemical performance of bismuth(III) fluoride in a fluoride shuttle battery. Chem. Phys. Lett. 755, 137785 (2020).
Liu, Q. et al. Rechargeable anion-shuttle batteries for low-cost energy storage. Chem 7, 1993–2021 (2021).
Gschwind, F., Euchner, H. & Rodriguez-Garcia, G. Chloride ion battery review: theoretical calculations, state of the art, safety, toxicity, and an outlook towards future developments. Eur. J. Inorg. Chem. 2017, 2784–2799 (2017).
Zhao, X., Zhao-Karger, Z., Fichtner, M. & Shen, X. Halide-based materials and chemistry for rechargeable batteries. Angew. Chem. Int. Ed. 59, 5902–5949 (2020).
Gao, P. et al. VOCl as a cathode for rechargeable chloride ion batteries. Angew. Chem. Int. Ed. 128, 4357–4362 (2016).
Chen, F., Leong, Z. Y. & Yang, H. Y. An aqueous rechargeable chloride ion battery. Energy Storage Mater. 7, 189–194 (2017).
Liang, G. et al. Commencing mild Ag–Zn batteries with long-term stability and ultra-flat voltage platform. Energy Storage Mater. 25, 86–92 (2020).
Wu, L., Chen, Z. & Ma, X. Chloride ion conducting polymer electrolytes based on cross-linked PMMA-PP14Cl-PP14TFSI ion gels for chloride ion batteries. Int. J. Electrochem. Sci. 14, 2414–2421 (2019).
Zhao, X. et al. Chloride ion battery: a new member in the rechargeable battery family. J. Power Sources 245, 706–711 (2014).
Zhao, X. et al. Carbon incorporation effects and reaction mechanism of FeOCl cathode materials for chloride ion batteries. Sci. Rep. 6, 19448 (2016).
Yu, T., Zhao, X., Ma, L. & Shen, X. Intercalation and electrochemical behaviors of layered FeOCl cathode material in chloride ion battery. Mater. Res. Bull. 96, 485–490 (2017).
Yu, T. et al. Nanoconfined iron oxychloride material as a high-performance cathode for rechargeable chloride ion batteries. ACS Energy Lett. 2, 2341–2348 (2017).
Lakshmi, K. P., Janas, K. J. & Shaijumon, M. M. Antimony oxychloride embedded graphene nanocomposite as efficient cathode material for chloride ion batteries. J. Power Sources 433, 126685 (2019).
Hu, X. et al. Electrochemical performance of Sb4O5Cl2 as a new anode material in aqueous chloride-ion battery. ACS Appl. Mater. Interfaces 11, 9144–9148 (2019).
Yin, Q. et al. CoFe–Cl layered double hydroxide: a new cathode material for high-performance chloride ion batteries. Adv. Funct. Mater. 29, 1900983 (2019).
Yin, Q. et al. A new family of rechargeable batteries based on halide ions shuttling. Chem. Eng. J. 389, 124376 (2020).
Zhao, X. et al. Developing polymer cathode material for the chloride ion battery. ACS Appl. Mater. Interfaces 9, 2535–2540 (2017).
Yang, R., Yu, T. & Zhao, X. Polypyrrole-coated iron oxychloride cathode material with improved cycling stability for chloride ion batteries. J. Alloy. Compd. 788, 407–412 (2019).
Yu, T. et al. Polyaniline-intercalated FeOCl cathode material for chloride-ion batteries. ChemElectroChem 6, 1761–1767 (2019).
Cadiou, V. et al. Pairing cross-linked polyviologen with aromatic amine host structure for anion shuttle rechargeable batteries. ChemSusChem 13, 2345–2353 (2020).
Yamaguchi, K. et al. Influence of the structure of the anion in an ionic liquid electrolyte on the electrochemical performance of a silicon negative electrode for a lithium-ion battery. J. Power Sources 338, 103–107 (2017).
Eshetu, G. G. et al. Impact of the electrolyte salt anion on the solid electrolyte interphase formation in sodium ion batteries. Nano Energy 55, 327–340 (2019).
Wang, H. et al. Efficient lithium metal cycling over a wide range of pressures from an anion-derived solid-electrolyte interphase framework. ACS Energy Lett. 6, 816–825 (2021).
Wang, Z. et al. An anion-tuned solid electrolyte interphase with fast ion transfer kinetics for stable lithium anodes. Adv. Energy Mater. 10, 1903843 (2020).
Wang, Z. et al. Highly concentrated dual-anion electrolyte for non-flammable high-voltage Li-metal batteries. Energy Storage Mater. 30, 228–237 (2020).
Yao, Y. et al. Regulating interfacial chemistry in lithium‐ion batteries by a weakly solvating electrolyte. Angew. Chem. Int. Ed. 133, 4136–4143 (2021).
Ding, J. F. et al. Non-solvating and low-dielectricity cosolvent for anion-derived solid electrolyte interphases in lithium metal batteries. Angew. Chem. Int. Ed. 60, 11442–11447 (2021).
Yan, C. et al. Nucleation and growth mechanism of anion‐derived solid electrolyte interphase in rechargeable batteries. Angew. Chem. Int. Ed. 133, 8602–8606 (2021). This work reveals the nucleation and growth mechanism of anion-derived solid electrolyte interphase film.
Tang, K. et al. A novel regulation strategy of solid electrolyte interphase based on anion-solvent coordination for magnesium metal anode. Small 16, 2005424 (2020).
Tang, K. et al. A stable solid electrolyte interphase for magnesium metal anode evolved from a bulky anion lithium salt. Adv. Mater. 32, 1904987 (2020).
Attias, R. et al. Anion effects on cathode electrochemical activity in rechargeable magnesium batteries: a case study of V2O5. ACS Energy Lett. 4, 209–214 (2019).
Zhang, W. et al. A ‘cation–anion regulation’ synergistic anode host for dendrite-free lithium metal batteries. Sci. Adv. 4, eaar4410 (2018).
Connell, J. G. et al. Anion association strength as a unifying descriptor for the reversibility of divalent metal deposition in nonaqueous electrolytes. ACS Appl. Mater. Interfaces 12, 36137–36147 (2020).
Tuerxun, F. et al. Effect of interaction among magnesium ions, anion, and solvent on kinetics of the magnesium deposition process. J. Phys. Chem. C 124, 28510–28519 (2020).
Huang, Z. et al. Ni3S2/Ni nanosheet arrays for high-performance flexible zinc hybrid batteries with evident two-stage charge and discharge processes. J. Mater. Chem. A 7, 18915–18924 (2019).
Li, X. et al. Activating the I/I+ redox couple in an aqueous I2-Zn battery to achieve a high voltage plateau. Energy Environ. Sci. 14, 407–413 (2021).
Yang, Q. et al. Hydrogen-substituted graphdiyne ion tunnels directing concentration redistribution for commercial-grade dendrite-free zinc anodes. Adv. Mater. 32, 2001755 (2020).
Yuan, D. et al. Anion texturing towards dendrite‐free Zn anode for aqueous rechargeable batteries. Angew. Chem. Int. Ed. 133, 7289–7295 (2021). This work demonstrates the important role of anions on the induced deposition of metal ions.
Niu, C. et al. Anion-regulated solid polymer electrolyte enhances the stable deposition of lithium ion for lithium metal batteries. J. Power Sources 417, 70–75 (2019).
Ma, C. et al. A borate decorated anion-immobilized solid polymer electrolyte for dendrite-free, long-life Li metal batteries. J. Mater. Chem. A 7, 19970–19976 (2019).
Hu, J. et al. The role of anions on the Helmholtz plane for the solid–liquid interface in aqueous rechargeable lithium batteries. Nano Energy 74, 104864 (2020).
Von Wald Cresce, A. et al. Anion solvation in carbonate-based electrolytes. J. Phys. Chem. C 119, 27255–27264 (2015).
Peng, J. et al. Multinuclear magnetic resonance investigation of cation–anion and anion–solvent interactions in carbonate electrolytes. J. Power Sources 399, 215–222 (2018).
Lau, K.-C. et al. Widening electrochemical window of Mg salt by weakly coordinating perfluoroalkoxyaluminate anion for Mg battery electrolyte. J. Electrochem. Soc. 166, A1510–A1519 (2019).
Fadel, E. R. et al. Role of solvent–anion charge transfer in oxidative degradation of battery electrolytes. Nat. Commun. 10, 3360 (2019). This work demonstrates that the solvation behaviours of anions greatly affect the electrochemical stability of electrolyte.
Yu, D. et al. Anion solvation regulation enables long cycle stability of graphite cathodes. ACS Energy Lett. 6, 949–958 (2021).
Jiang, B. et al. The anion effect on Li+ ion coordination structure in ethylene carbonate solutions. J. Phys. Chem. Lett. 7, 3554–3559 (2016).
Zhang, X. Q. et al. Regulating anions in the solvation sheath of lithium ions for stable lithium metal batteries. ACS Energy Lett. 4, 411–416 (2019).
Yu, Z. et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 5, 526–533 (2020).
Jiang, C. et al. Batteries regulating the solvation sheath of Li ions by using hydrogen bonds for highly stable lithium–metal anodes research articles. Angew. Chem. Int. Ed. 60, 2–11 (2021).
Suo, L. et al. ‘Water-in-salt’ electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Chang, Z. et al. Environmental science beyond the concentrated electrolyte: further depleting solvent molecules within a Li+ solvation sheath to stabilize high-energy-density lithium. Energy Environ. Sci. 13, 4122–4131 (2020).
Acknowledgements
This work was supported by the InnoHK Project (Project 1.3 — Flexible and Stretchable Technologies (FAST) for monitoring of CVD risk factors: sensing and applications and Project 1.4 — Flexible and Stretchable Technologies (FAST) for monitoring of CVD risk factors: soft battery and self-powered, flexible medical devices) at the Hong Kong Centre for Cerebro-cardiovascular Health Engineering (COCHE). X.J. thanks the US National Science Foundation for the financial support with the award no. DMR 2221645.
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation and reviewing of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Ho Seok Park and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Z., Li, X., Chen, Z. et al. Anion chemistry in energy storage devices. Nat Rev Chem 7, 616–631 (2023). https://doi.org/10.1038/s41570-023-00506-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00506-w
This article is cited by
-
Water-stable boroxine structure with dynamic covalent bonds
Nature Communications (2024)
-
Understanding steric hindrance effect of solvent molecule in localized high-concentration electrolyte for lithium metal batteries
Carbon Neutrality (2023)