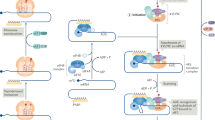
Abstract
Cells can rapidly adjust their proteomes in dynamic environments by regulating mRNA translation. There is mounting evidence that dysregulation of mRNA translation supports the survival and adaptation of cancer cells, which has stimulated clinical interest in targeting elements of the translation machinery and, in particular, components of the eukaryotic initiation factor 4F (eIF4F) complex such as eIF4E. However, the effect of targeting mRNA translation on infiltrating immune cells and stromal cells in the tumour microenvironment (TME) has, until recently, remained unexplored. In this Perspective article, we discuss how eIF4F-sensitive mRNA translation controls the phenotypes of key non-transformed cells in the TME, with an emphasis on the underlying therapeutic implications of targeting eIF4F in cancer. As eIF4F-targeting agents are in clinical trials, we propose that a broader understanding of their effect on gene expression in the TME will reveal unappreciated therapeutic vulnerabilities that could be used to improve the efficacy of existing cancer therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Aebersold, R. & Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–355 (2016).
Schwanhausser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011). This comprehensive work uses parallel metabolic pulse labelling to quantify protein and mRNA levels from the same cell at the steady state and is among the earliest to highlight the extent to which post-transcriptional regulation of gene expression shapes the proteome.
Zhang, B. et al. Proteogenomic characterization of human colon and rectal cancer. Nature 513, 382–387 (2014).
Mun, D. G. et al. Proteogenomic characterization of human early-onset gastric cancer. Cancer Cell 35, 111–124.e10 (2019).
Tang, W. et al. Integrated proteotranscriptomics of breast cancer reveals globally increased protein–mRNA concordance associated with subtypes and survival. Genome Med. 10, 94 (2018).
Bartish, M. et al. MNK2 governs the macrophage antiinflammatory phenotype. Proc. Natl Acad. Sci. USA 117, 27556–27565 (2020). This study is the first to perform transcriptome-wide profiling of mRNA translation in a primary cell type isolated from the murine breast tumour microenvironment and implicate alterations in mRNA translation as a dominant mode of gene expression regulation in tumour-associated macrophages.
Fabbri, L., Chakraborty, A., Robert, C. & Vagner, S. The plasticity of mRNA translation during cancer progression and therapy resistance. Nat. Rev. Cancer 21, 558–577 (2021).
Bhat, M. et al. Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 14, 261–278 (2015).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 11, 3801 (2020).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Su, S. et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell 175, 442–457.e23 (2018).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Mariathasan, S. et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Tang, T. et al. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal. Transduct. Target. Ther. 6, 72 (2021).
Rolny, C. et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19, 31–44 (2011). This paper illustrates that normalizing the tumour vasculature through modulating the phenotype of tumour-associated macrophages impedes tumour growth and distant metastasis.
De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017).
Huang, F. et al. Inhibiting the MNK1/2-eIF4E axis impairs melanoma phenotype switching and potentiates antitumor immune responses. J. Clin. Invest. https://doi.org/10.1172/JCI140752 (2021). This study implicates the MNK-eIF4E axis in regulating the translation of CCL5 in cancer cells and, separately, the downstream expression of PDL1 on dendritic cells and myeloid-derived suppressor cells.
Sandri, B. J. et al. Distinct cancer-promoting stromal gene expression depending on lung function. Am. J. Respir. Crit. Care Med. 200, 348–358 (2019). This study uses a multi-omics approach on patient-derived non-cancerous tissue from patients with chronic obstructive pulmonary disease with or without lung cancer to identify distinct translationally regulated gene expression signatures acting in the lung stroma that promote cancer initiation.
Hurst, K. E. et al. Remodeling translation primes CD8(+) T-cell antitumor immunity. Cancer Immunol. Res. 8, 587–595 (2020).
De Ponte Conti, B. et al. mTOR-dependent translation drives tumor infiltrating CD8(+) effector and CD4(+) Treg cells expansion. eLife https://doi.org/10.7554/eLife.69015 (2021). This paper is the first to directly compare the rate of translation in splenic T cells and tumour-infiltrating T cells, demonstrating that T cell translation is upregulated in the tumour context.
Guo, Q. et al. The MNK1/2-eIF4E axis supports immune suppression and metastasis in postpartum breast cancer. Cancer Res. 81, 3876–3889 (2021).
Robichaud, N. et al. Translational control in the tumor microenvironment promotes lung metastasis: phosphorylation of eIF4E in neutrophils. Proc. Natl Acad. Sci. USA 115, E2202–E2209 (2018). This is the first study to use the S209A phospho-eIF4E-deficient mouse model to study how phospho-eIF4E deficiency in cells of the tumour immune microenvironment impacts on the ability of syngeneic phospho-eIF4E competent breast cancer cells to metastasize.
Preston, S. E. J. et al. Phosphorylation of eIF4E in the stroma drives the production and spatial organisation of collagen type I in the mammary gland. Matrix Biol. https://doi.org/10.1016/j.matbio.2022.07.003 (2022).
Cerezo, M., Robert, C., Liu, L. & Shen, S. The role of mRNA translational control in tumor immune escape and immunotherapy resistance. Cancer Res. 81, 5596–5604 (2021).
Sonenberg, N., Morgan, M. A., Merrick, W. C. & Shatkin, A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5′-terminal cap in mRNA. Proc. Natl Acad. Sci. USA 75, 4843–4847 (1978).
Jackson, R. J., Hellen, C. U. & Pestova, T. V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 (2010).
Hershey, J. W. B., Sonenberg, N. & Mathews, M. B. Principles of translational control. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a032607 (2019).
Joshi, B., Yan, R. & Rhoads, R. E. In vitro synthesis of human protein synthesis initiation factor 4 gamma and its localization on 43 and 48S initiation complexes. J. Biol. Chem. 269, 2048–2055 (1994).
Mader, S., Lee, H., Pause, A. & Sonenberg, N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol. Cell Biol. 15, 4990–4997 (1995).
Haghighat, A., Mader, S., Pause, A. & Sonenberg, N. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 14, 5701–5709 (1995).
Gingras, A. C. et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 15, 2852–2864 (2001).
Gingras, A. C. et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 13, 1422–1437 (1999).
Pause, A. et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371, 762–767 (1994).
von Manteuffel, S. R., Gingras, A. C., Ming, X. F., Sonenberg, N. & Thomas, G. 4E-BP1 phosphorylation is mediated by the FRAP–p70s6k pathway and is independent of mitogen-activated protein kinase. Proc. Natl Acad. Sci. USA 93, 4076–4080 (1996).
Burnett, P. E., Barrow, R. K., Cohen, N. A., Snyder, S. H. & Sabatini, D. M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl Acad. Sci. USA 95, 1432–1437 (1998).
Ray, B. K. et al. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J. Biol. Chem. 260, 7651–7658 (1985).
Duncan, R., Milburn, S. C. & Hershey, J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J. Biol. Chem. 262, 380–388 (1987).
Kovalski, J. R., Kuzuoglu-Ozturk, D. & Ruggero, D. Protein synthesis control in cancer: selectivity and therapeutic targeting. EMBO J. 41, e109823 (2022).
Piecyk, M. et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 19, 4154–4163 (2000).
Grosset, C. et al. In vivo studies of translational repression mediated by the granulocyte-macrophage colony-stimulating factor AU-rich element. J. Biol. Chem. 279, 13354–13362 (2004).
Dixon, D. A. et al. Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. J. Exp. Med. 198, 475–481 (2003).
Schott, J. et al. Translational regulation of specific mRNAs controls feedback inhibition and survival during macrophage activation. PLoS Genet. 10, e1004368 (2014).
Vyas, K. et al. Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in gamma interferon-activated monocytes. Mol. Cell Biol. 29, 458–470 (2009).
Liepelt, A. et al. Translation control of TAK1 mRNA by hnRNP K modulates LPS-induced macrophage activation. RNA 20, 899–911 (2014).
Moore, M. J. et al. ZFP36 RNA-binding proteins restrain T cell activation and anti-viral immunity. eLife 7, e33057 (2018).
Bell, S. E. et al. The RNA binding protein Zfp36l1 is required for normal vascularisation and post-transcriptionally regulates VEGF expression. Dev. Dyn. 235, 3144–3155 (2006).
Ray, P. S. & Fox, P. L. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 26, 3360–3372 (2007).
Ray, P. S. et al. A stress-responsive RNA switch regulates VEGFA expression. Nature 457, 915–919 (2009).
Julio, A. R. & Backus, K. M. New approaches to target RNA binding proteins. Curr. Opin. Chem. Biol. 62, 13–23 (2021).
Larsson, O. et al. Apoptosis resistance downstream of eIF4E: posttranscriptional activation of an anti-apoptotic transcript carrying a consensus hairpin structure. Nucleic Acids Res. 34, 4375–4386 (2006).
Larsson, O. et al. Eukaryotic translation initiation factor 4E induced progression of primary human mammary epithelial cells along the cancer pathway is associated with targeted translational deregulation of oncogenic drivers and inhibitors. Cancer Res. 67, 6814–6824 (2007).
Gandin, V. et al. nanoCAGE reveals 5′ UTR features that define specific modes of translation of functionally related MTOR-sensitive mRNAs. Genome Res. 26, 636–648 (2016). This paper cautions on how subtle technical differences between polysome and ribosome profiling methods for studying transcriptome-wide translation can lead to divergent interpretation of the data obtained.
Furic, L. et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc. Natl Acad. Sci. USA 107, 14134–14139 (2010). This paper reports the generation of the S209A phospho-eIF4E deficient mouse model, a key tool for studying the consequences of disrupted eIF4E phosphorylation in cancer and other contexts.
Wolfe, A. L. et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature 513, 65–70 (2014).
Ramirez-Valle, F., Braunstein, S., Zavadil, J., Formenti, S. C. & Schneider, R. J. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J. Cell Biol. 181, 293–307 (2008).
Kozak, M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15, 8125–8148 (1987).
Koromilas, A. E., Lazaris-Karatzas, A. & Sonenberg, N. mRNAs containing extensive secondary structure in their 5′ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J. 11, 4153–4158 (1992).
Svitkin, Y. V. et al. The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5′ secondary structure. RNA 7, 382–394 (2001).
Rubio, C. A. et al. Transcriptome-wide characterization of the eIF4A signature highlights plasticity in translation regulation. Genome Biol. 15, 476 (2014).
Waldron, J. A., Raza, F. & Le Quesne, J. eIF4A alleviates the translational repression mediated by classical secondary structures more than by G-quadruplexes. Nucleic Acids Res. 46, 3075–3087 (2018).
Hsieh, A. C. et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485, 55–61 (2012).
Eliseeva, I. et al. In silico motif analysis suggests an interplay of transcriptional and translational control in mTOR response. Translation 1, e27469 (2013).
Iwasaki, S., Floor, S. N. & Ingolia, N. T. Rocaglates convert DEAD-box protein eIF4A into a sequence-selective translational repressor. Nature 534, 558–561 (2016).
Chu, J. et al. Rocaglates induce gain-of-function alterations to eIF4A and eIF4F. Cell Rep. 30, 2481–2488 e2485 (2020).
Ueda, T., Watanabe-Fukunaga, R., Fukuyama, H., Nagata, S. & Fukunaga, R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell Biol. 24, 6539–6549 (2004). This paper reports the generation of the MNK1 and MNK2 double knockout mouse model, a key in vivo tool for studying the consequences of disrupted MNK signalling in cancer and other contexts.
Lama, D. & Verma, C. S. Deciphering the mechanistic effects of eIF4E phosphorylation on mRNA-cap recognition. Protein Sci. 29, 1373–1386 (2020).
Scheper, G. C. et al. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J. Biol. Chem. 277, 3303–3309 (2002).
Slepenkov, S. V., Darzynkiewicz, E. & Rhoads, R. E. Stopped-flow kinetic analysis of eIF4E and phosphorylated eIF4E binding to cap analogs and capped oligoribonucleotides: evidence for a one-step binding mechanism. J. Biol. Chem. 281, 14927–14938 (2006).
Zuberek, J. et al. Phosphorylation of eIF4E attenuates its interaction with mRNA 5′ cap analogs by electrostatic repulsion: intein-mediated protein ligation strategy to obtain phosphorylated protein. RNA 9, 52–61 (2003).
de Breyne, S., Yu, Y., Unbehaun, A., Pestova, T. V. & Hellen, C. U. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc. Natl Acad. Sci. USA 106, 9197–9202 (2009).
Hundsdoerfer, P., Thoma, C. & Hentze, M. W. Eukaryotic translation initiation factor 4GI and p97 promote cellular internal ribosome entry sequence-driven translation. Proc. Natl Acad. Sci. USA 102, 13421–13426 (2005).
Braunstein, S. et al. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell 28, 501–512 (2007).
Silvera, D. et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat. Cell Biol. 11, 903–908 (2009).
Akirtava, C., May, G. E. & McManus, C. J. False-positive IRESes from Hoxa9 and other genes resulting from errors in mammalian 5′ UTR annotations. Proc. Natl Acad. Sci. USA 119, e2122170119 (2022).
Stein, I. et al. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol. Cell Biol. 18, 3112–3119 (1998).
Lang, K. J., Kappel, A. & Goodall, G. J. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol. Biol. Cell 13, 1792–1801 (2002).
Stoneley, M., Paulin, F. E., Le Quesne, J. P., Chappell, S. A. & Willis, A. E. C-Myc 5′ untranslated region contains an internal ribosome entry segment. Oncogene 16, 423–428 (1998).
Sherrill, K. W., Byrd, M. P., Van Eden, M. E. & Lloyd, R. E. BCL-2 translation is mediated via internal ribosome entry during cell stress. J. Biol. Chem. 279, 29066–29074 (2004).
Vagner, S. et al. Alternative translation of human fibroblast growth factor 2 mRNA occurs by internal entry of ribosomes. Mol. Cell Biol. 15, 35–44 (1995).
Rosenwald, I. B. et al. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J. Biol. Chem. 270, 21176–21180 (1995).
De Benedetti, A., Joshi, B., Graff, J. & Zimmer, S. CHO cells transformed by the translation factor eIF-4E display increased c-myc expression, but require overexpression of Max for tumorigenicity. Mol. Cell Differ. 2, 347–371 (1994).
Lin, C. J., Cencic, R., Mills, J. R., Robert, F. & Pelletier, J. c-Myc and eIF4F are components of a feedforward loop that links transcription and translation. Cancer Res. 68, 5326–5334 (2008).
Wendel, H. G. et al. Dissecting eIF4E action in tumorigenesis. Genes. Dev. 21, 3232–3237 (2007).
Robichaud, N. et al. Phosphorylation of eIF4E promotes EMT and metastasis via translational control of SNAIL and MMP-3. Oncogene 34, 2032–2042 (2015).
Kevil, C. G. et al. Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E: implications for tumor angiogenesis. Int. J. Cancer 65, 785–790 (1996).
Cunningham, J. T., Moreno, M. V., Lodi, A., Ronen, S. M. & Ruggero, D. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell 157, 1088–1103 (2014).
Xu, Y. et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med. 25, 301–311 (2019). This paper shows that PDL1 expression is controlled at the level of mRNA translation in a model of aggressive liver cancer and demonstrates that MNK inhibition prolongs the survival of and downregulates PDL1 expression in the cancer cells of tumour-bearing mice.
Lazaris-Karatzas, A., Montine, K. S. & Sonenberg, N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 345, 544–547 (1990).
Lazaris-Karatzas, A. & Sonenberg, N. The mRNA 5′ cap-binding protein, eIF-4E, cooperates with v-myc or E1A in the transformation of primary rodent fibroblasts. Mol. Cell Biol. 12, 1234–1238 (1992).
Fukuchi-Shimogori, T. et al. Malignant transformation by overproduction of translation initiation factor eIF4G. Cancer Res. 57, 5041–5044 (1997).
Truitt, M. L. et al. Differential requirements for eIF4E dose in normal development and cancer. Cell 162, 59–71 (2015). This study uses a haploinsufficient eIF4E mouse model to illustrate the relationship between eIF4E protein levels and tumour development, emphasizing the dependency of oncogenic transformation on elevated levels of eIF4F-mediated translation.
Ruggero, D. et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat. Med. 10, 484–486 (2004).
Zheng, J. & Gao, P. Toward normalization of the tumor microenvironment for cancer therapy. Integr. Cancer Ther. 18, 1534735419862352 (2019).
Sanmamed, M. F. & Chen, L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 175, 313–326 (2018).
Cerezo, M. et al. Translational control of tumor immune escape via the eIF4F–STAT1–PD-L1 axis in melanoma. Nat. Med. 24, 1877–1886 (2018). This study is the first to implicate eIF4F-driven translation inhibition as an anti-tumour therapy by showing how eIF4A, via control of STAT1 translation, regulates the expression of immuno-suppressive PDL1 on cancer cells.
Oh, S. A. et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 1, 681–691 (2020).
Peng, Q. et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 11, 4835 (2020).
Pham, T. N. et al. Inhibition of MNKs promotes macrophage immunosuppressive phenotype to limit CD8+ T cell antitumor immunity. JCI Insight https://doi.org/10.1172/jci.insight.152731 (2022).
Wang, X. et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015).
Han, D. et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274 (2019).
Clarke, C. J. et al. The initiator methionine tRNA drives secretion of type II collagen from stromal fibroblasts to promote tumor growth and angiogenesis. Curr. Biol. 26, 755–765 (2016).
Manfrini, N. et al. Ribosome profiling unveils translational regulation of metabolic enzymes in primary CD4(+) Th1 cells. Dev. Comp. Immunol. 109, 103697 (2020). This study is the first to perform ribosome profiling on primary human T cells and shows that the expression of many metabolic enzymes is regulated at the level of translation.
Liang, S. et al. Polysome-profiling in small tissue samples. Nucleic Acids Res. 46, e3 (2018). This paper improves on the original polysome profiling protocol to allow for efficient polysome-bound mRNA extraction from low input samples, such as primary isolated cells or banked tissue.
Lahmar, Q. et al. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim. Biophys. Acta 1865, 23–34 (2016).
Mahoney, T. S. et al. Cell adhesion regulates gene expression at translational checkpoints in human myeloid leukocytes. Proc. Natl Acad. Sci. USA 98, 10284–10289 (2001).
Fox, R. et al. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J. 26, 505–515 (2007).
Amano, M. et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246–20249 (1996).
Pinner, S. & Sahai, E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat. Cell Biol. 10, 127–137 (2008).
Kawai, T. et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167, 5887–5894 (2001).
Toshchakov, V. et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat. Immunol. 3, 392–398 (2002).
Martinez, F. O., Gordon, S., Locati, M. & Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 (2006).
Naler, L. B. et al. Epigenomic and transcriptomic analyses reveal differences between low-grade inflammation and severe exhaustion in LPS-challenged murine monocytes. Commun. Biol. 5, 102 (2022).
Kittan, N. A. et al. Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS ONE 8, e78045 (2013).
Piccolo, V. et al. Opposing macrophage polarization programs show extensive epigenomic and transcriptional cross-talk. Nat. Immunol. 18, 530–540 (2017).
Su, X. et al. Interferon-gamma regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat. Immunol. 16, 838–849 (2015).
Herdy, B. et al. Translational control of the activation of transcription factor NF-kappaB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat. Immunol. 13, 543–550 (2012).
Bao, Y. et al. Brd4 modulates the innate immune response through Mnk2-eIF4E pathway-dependent translational control of IκBα. Proc. Natl Acad. Sci. USA 114, E3993–E4001 (2017).
Wallerius, M. et al. Guidance molecule SEMA3A restricts tumor growth by differentially regulating the proliferation of tumor-associated macrophages. Cancer Res. 76, 3166–3178 (2016).
Petty, A. J. et al. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J. Clin. Invest. 129, 5151–5162 (2019).
Ruffell, B. et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637 (2014).
DeNardo, D. G. et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1, 54–67 (2011).
Zhu, Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014).
Candido, J. B. et al. CSF1R(+) macrophages sustain pancreatic tumor growth through T cell suppression and maintenance of key gene programs that define the squamous subtype. Cell Rep. 23, 1448–1460 (2018).
Xu, H. et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 13, 642–650 (2012).
William, M. et al. Translational repression of Ccl5 and Cxcl10 by 4E-BP1 and 4E-BP2 restrains the ability of mouse macrophages to induce migration of activated T cells. Eur. J. Immunol. 49, 1200–1212 (2019).
William, M. et al. eIF4E-binding proteins 1 and 2 limit macrophage anti-inflammatory responses through translational repression of IL-10 and cyclooxygenase-2. J. Immunol. 200, 4102–4116 (2018).
Roche, P. A. & Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 15, 203–216 (2015).
Hipolito, V. E. B. et al. Enhanced translation expands the endo-lysosome size and promotes antigen presentation during phagocyte activation. PLoS Biol. 17, e3000535 (2019).
Lelouard, H. et al. Regulation of translation is required for dendritic cell function and survival during activation. J. Cell Biol. 179, 1427–1439 (2007).
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020).
Mantovani, A., Allavena, P., Marchesi, F. & Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21, 799–820 (2022).
Bjur, E. et al. Distinct translational control in CD4+ T cell subsets. PLoS Genet. 9, e1003494 (2013).
Aviner, R. The science of puromycin: from studies of ribosome function to applications in biotechnology. Comput. Struct. Biotechnol. J. 18, 1074–1083 (2020).
Biswas, B. et al. Differential effects on the translation of immune-related alternatively polyadenylated mRNAs in melanoma and T cells by eIF4A inhibition. Cancers https://doi.org/10.3390/cancers14051177 (2022).
Roos, D. & Loos, J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes: II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp. Cell Res. 77, 127–135 (1973).
van der Windt, G. J. W. & Pearce, E. L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 249, 27–42 (2012).
Chapman, N. M. & Chi, H. Hallmarks of T-cell exit from quiescence. Cancer Immunol. Res. 6, 502–508 (2018).
Ricciardi, S. et al. The translational machinery of human CD4(+) T cells is poised for activation and controls the switch from quiescence to metabolic remodeling. Cell Metab. 28, 895–906.e95 (2018). This article demonstrates that pre-accumulated mRNAs are translated through the eIF4F complex upon T cell activation to promote metabolic rewiring.
Wolf, T. et al. Dynamics in protein translation sustaining T cell preparedness. Nat. Immunol. 21, 927–937 (2020).
Nelson, D. L., Lehninger, A. L. & Cox, M. M. Lehninger Principles of Biochemistry (Macmillan, 2008).
Garibaldi, A., Carranza, F. & Hertel, K. J. Isolation of newly transcribed RNA using the metabolic label 4-thiouridine. Methods Mol. Biol. 1648, 169–176 (2017).
Davari, K. et al. Rapid genome-wide recruitment of RNA polymerase II drives transcription, splicing, and translation events during T cell responses. Cell Rep. 19, 643–654 (2017).
Liedmann, S. et al. Localization of a TORC1-eIF4F translation complex during CD8(+) T cell activation drives divergent cell fate. Mol. Cell 82, 2401–2414.e9 (2022). This study in T cells demonstrates that the localization of the eIF4F complex in the proximal pole during cell division impacts daughter cell lineage identity, through the upregulation of the translation of c-myc.
Araki, K. et al. Translation is actively regulated during the differentiation of CD8(+) effector T cells. Nat. Immunol. 18, 1046–1057 (2017).
Scheu, S. et al. Activation of the integrated stress response during T helper cell differentiation. Nat. Immunol. 7, 644–651 (2006).
Nikolcheva, T. et al. A translational rheostat for RFLAT-1 regulates RANTES expression in T lymphocytes. J. Clin. Investig. 110, 119–126 (2002).
Aldinucci, D., Borghese, C. & Casagrande, N. The CCL5/CCR5 axis in cancer progression. Cancers https://doi.org/10.3390/cancers12071765 (2020).
Gigoux, M. et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc. Natl Acad. Sci. USA 106, 20371–20376 (2009).
Gigoux, M. et al. Inducible costimulator facilitates T-dependent B cell activation by augmenting IL-4 translation. Mol. Immunol. 59, 46–54 (2014).
Cook, K. D. & Miller, J. TCR-dependent translational control of GATA-3 enhances Th2 differentiation. J. Immunol. 185, 3209–3216 (2010).
Chow, A., Perica, K., Klebanoff, C. A. & Wolchok, J. D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 19, 775–790 (2022).
Yost, K. E., Chang, H. Y. & Satpathy, A. T. Recruiting T cells in cancer immunotherapy. Science 372, 130–131 (2021).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176, 1248–1264 (2019).
Arcondeguy, T., Lacazette, E., Millevoi, S., Prats, H. & Touriol, C. VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res. 41, 7997–8010 (2013).
Huez, I. et al. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol. Cell Biol. 18, 6178–6190 (1998).
Kraggerud, S. M., Sandvik, J. A. & Pettersen, E. O. Regulation of protein synthesis in human cells exposed to extreme hypoxia. Anticancer. Res. 15, 683–686 (1995).
Liu, L. et al. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 21, 521–531 (2006).
Thomas, J. D. & Johannes, G. J. Identification of mRNAs that continue to associate with polysomes during hypoxia. RNA 13, 1116–1131 (2007).
Koumenis, C. et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol. Cell Biol. 22, 7405–7416 (2002).
Bornes, S. et al. Translational induction of VEGF internal ribosome entry site elements during the early response to ischemic stress. Circ. Res. 100, 305–308 (2007).
Byrnes, K. et al. High eIF4E, VEGF, and microvessel density in stage I to III breast cancer. Ann. Surg. 243, 684–690 (2006).
Yang, S. X., Hewitt, S. M., Steinberg, S. M., Liewehr, D. J. & Swain, S. M. Expression levels of eIF4E, VEGF, and cyclin D1, and correlation of eIF4E with VEGF and cyclin D1 in multi-tumor tissue microarray. Oncol. Rep. 17, 281–287 (2007).
Chung, J., Bachelder, R. E., Lipscomb, E. A., Shaw, L. M. & Mercurio, A. M. Integrin (α6β4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J. Cell Biol. 158, 165–174 (2002).
Korneeva, N. L. et al. Mnk mediates integrin alpha6beta4-dependent eIF4E phosphorylation and translation of VEGF mRNA. Mol. Cancer Res. 8, 1571–1578 (2010).
Guba, M. et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat. Med. 8, 128–135 (2002).
Chen, S. et al. Preclinical evidence that MNK/eIF4E inhibition by cercosporamide enhances the response to antiangiogenic TKI and mTOR inhibitor in renal cell carcinoma. Biochem. Biophys. Res. Commun. 530, 142–148 (2020).
Liu, Y., Sun, L., Su, X. & Guo, S. Inhibition of eukaryotic initiation factor 4E phosphorylation by cercosporamide selectively suppresses angiogenesis, growth and survival of human hepatocellular carcinoma. Biomed. Pharmacother. 84, 237–243 (2016).
Sato, T. N., Qin, Y., Kozak, C. A. & Audus, K. L. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc. Natl Acad. Sci. USA 90, 9355–9358 (1993).
Park, E. H., Lee, J. M., Blais, J. D., Bell, J. C. & Pelletier, J. Internal translation initiation mediated by the angiogenic factor Tie2. J. Biol. Chem. 280, 20945–20953 (2005).
Graff, J. R. et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J. Clin. Investig. 117, 2638–2648 (2007).
Schmid, D. I. et al. Translational control of JunB, an AP-1 transcription factor, in activated human endothelial cells. J. Cell Biochem. 114, 1519–1528 (2013).
Hughes, R. et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 75, 3479–3491 (2015).
Lewis, J. S., Landers, R. J., Underwood, J. C., Harris, A. L. & Lewis, C. E. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J. Pathol. 192, 150–158 (2000).
De Palma, M. et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226 (2005).
Venneri, M. A. et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 109, 5276–5285 (2007).
Phan, S. H. Genesis of the myofibroblast in lung injury and fibrosis. Proc. Am. Thorac. Soc. 9, 148–152 (2012).
Rogler, G. et al. Differential activation of cytokine secretion in primary human colonic fibroblast/myofibroblast cultures. Scand. J. Gastroenterol. 36, 389–398 (2001).
Shimada, M. et al. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J. Immunol. 168, 861–868 (2002).
Darby, I. A., Zakuan, N., Billet, F. & Desmouliere, A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol. Life Sci. 73, 1145–1157 (2016).
Xia, H. et al. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J. Exp. Med. 205, 1659–1672 (2008).
Larsson, O. et al. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS ONE 3, e3220 (2008).
Parker, M. W. et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Invest. 124, 1622–1635 (2014).
Ropponen, K. et al. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 58, 342–347 (1998).
Anttila, M. A. et al. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 60, 150–155 (2000).
Auvinen, P. et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 156, 529–536 (2000).
Zhou, Z. H. et al. Reorganized collagen in the tumor microenvironment of gastric cancer and its association with prognosis. J. Cancer 8, 1466–1476 (2017).
Peng, D. H. et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat. Commun. 11, 4520 (2020).
Hsieh, A. C. et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell 17, 249–261 (2010).
Alain, T. et al. eIF4E/4E-BP ratio predicts the efficacy of mTOR targeted therapies. Cancer Res. 72, 6468–6476 (2012).
Guo, Q. et al. MNK1/NODAL signaling promotes invasive progression of breast ductal carcinoma in situ. Cancer Res. 79, 1646–1657 (2019).
Yang, W. et al. MNK1 signaling induces an ANGPTL4-mediated gene signature to drive melanoma progression. Oncogene 39, 3650–3665 (2020).
Zhan, Y. et al. MNK1/2 inhibition limits oncogenicity and metastasis of KIT-mutant melanoma. J. Clin. Invest. 127, 4179–4192 (2017).
Demosthenous, C. et al. Translation initiation complex eIF4F is a therapeutic target for dual mTOR kinase inhibitors in non-Hodgkin’s lymphoma. Oncotarget 6, 9488–9501 (2015).
Culjkovic-Kraljacic, B. et al. Combinatorial targeting of nuclear export and translation of RNA inhibits aggressive B-cell lymphomas. Blood 127, 858–868 (2016).
Martinez-Marignac, V. et al. Pharmacological targeting of eIF4E in primary CLL lymphocytes. Blood Cancer J. 3, e146 (2013).
Robert, F. et al. Translation initiation factor eIF4F modifies the dexamethasone response in multiple myeloma. Proc. Natl Acad. Sci. USA 111, 13421–13426 (2014).
Blázquez-Domingo, M., Grech, G. & Lindern, M. V. Translation initiation factor 4E inhibits differentiation of erythroid progenitors. Mol. Cell. Biol. 25, 8496–8506 (2005).
Forester, C. M. et al. Regulation of eIF4E guides a unique translational program to control erythroid maturation. Sci. Adv. 8, eadd3942 (2022).
Chiu, H. et al. Reduced eIF4E function impairs B-cell leukemia without altering normal B-lymphocyte function. iScience 24, 102748 (2021).
Chan, K. et al. eIF4A supports an oncogenic translation program in pancreatic ductal adenocarcinoma. Nat. Commun. 10, 5151 (2019).
Sanghvi, V. R. et al. NRF2 activation confers resistance to eIF4A inhibitors in cancer therapy. Cancers 13, 639 (2021).
Kuznetsov, G. et al. Potent in vitro and in vivo anticancer activities of des-methyl, des-amino pateamine A, a synthetic analogue of marine natural product pateamine A. Mol. Cancer Ther. 8, 1250–1260 (2009).
Campos, A. et al. Anti-tumour effects of elatol, a marine derivative compound obtained from red algae Laurencia microcladia. J. Pharm. Pharmacol. 64, 1146–1154 (2012).
Peters, T. L. et al. Target-based screening against eIF4A1 reveals the marine natural product elatol as a novel inhibitor of translation initiation with in vivo antitumor activity. Clin. Cancer Res. 24, 4256–4270 (2018).
Boussemart, L. et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature 513, 105–109 (2014).
Cencic, R. et al. Modifying chemotherapy response by targeted inhibition of eukaryotic initiation factor 4A. Blood Cancer J. 3, e128 (2013).
Nishida, Y. et al. Inhibition of translation initiation factor eIF4a inactivates heat shock factor 1 (HSF1) and exerts anti-leukemia activity in AML. Leukemia 35, 2469–2481 (2021).
Kogure, T. et al. Therapeutic potential of the translation inhibitor silvestrol in hepatocellular cancer. PLoS ONE 8, e76136 (2013).
Gerson-Gurwitz, A. et al. Zotatifin, an eIF4A-selective inhibitor, blocks tumor growth in receptor tyrosine kinase driven tumors. Front. Oncol. https://doi.org/10.3389/fonc.2021.766298 (2021).
Chen, L. et al. Tumor suppression by small molecule inhibitors of translation initiation. Oncotarget 3, 869–881 (2012).
Yi, T., Kabha, E., Papadopoulos, E. & Wagner, G. 4EGI-1 targets breast cancer stem cells by selective inhibition of translation that persists in CSC maintenance, proliferation and metastasis. Oncotarget 5, 6028–6037 (2014).
Wu, M., Zhang, C., Li, X. J., Liu, Q. & Wanggou, S. Anti-cancer effect of cap-translation inhibitor 4EGI-1 in human glioma U87 cells: involvement of mitochondrial dysfunction and ER stress. Cell Physiol. Biochem. 40, 1013–1028 (2016).
Cencic, R. et al. Reversing chemoresistance by small molecule inhibition of the translation initiation complex eIF4F. Proc. Natl Acad. Sci. USA 108, 1046–1051 (2011).
Kardos, G. R., Gowda, R., Dinavahi, S. S., Kimball, S. & Robertson, G. P. Salubrinal in combination with 4E1RCat synergistically impairs melanoma development by disrupting the protein synthetic machinery. Front. Oncol. https://doi.org/10.3389/fonc.2020.00834 (2020).
Feng, Y. et al. SBI-0640756 attenuates the growth of clinically unresponsive melanomas by disrupting the eIF4F translation initiation complex. Cancer Res. 75, 5211–5218 (2015).
Herzog, L.-o et al. Targeting eIF4F translation initiation complex with SBI-756 sensitises B lymphoma cells to venetoclax. Br. J. Cancer 124, 1098–1109 (2021).
Konicek, B. W. et al. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 71, 1849–1857 (2011).
Li, Z. et al. Inhibiting the MNK-eIF4E-β-catenin axis increases the responsiveness of aggressive breast cancer cells to chemotherapy. Oncotarget 8, 2906–2915 (2017).
Pham, T. N. D. et al. Induction of MNK kinase-dependent eIF4E phosphorylation by inhibitors targeting BET proteins limits efficacy of BET inhibitors. Mol. Cancer Ther. 18, 235–244 (2019).
Wen, Q. et al. CGP57380 enhances efficacy of RAD001 in non-small cell lung cancer through abrogating mTOR inhibition-induced phosphorylation of eIF4E and activating mitochondrial apoptotic pathway. Oncotarget 7, 27787–27801 (2016).
Kuzuoglu-Ozturk, D. et al. Revealing molecular pathways for cancer cell fitness through a genetic screen of the cancer translatome. Cell Rep. 35, 109321 (2021).
Lou, S., Balluff, B., Cleven, A. H. G., Bovee, J. & McDonnell, L. A. Prognostic metabolite biomarkers for soft tissue sarcomas discovered by mass spectrometry imaging. J. Am. Soc. Mass. Spectrom. 28, 376–383 (2017).
Gerdes, M. J. et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc. Natl Acad. Sci. USA 110, 11982–11987 (2013).
Lin, J. R. et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife https://doi.org/10.7554/eLife.31657 (2018).
Goltsev, Y. et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174, 968–981.e15 (2018).
Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014).
Karimi, E. et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 614, 555–563 (2023).
Sorin, M. et al. Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature 614, 548–554 (2023).
Angelo, M. et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 20, 436–442 (2014).
Keren, L. et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174, 1373–1387.e19 (2018).
Simonson, P. D., Valencia, I. & Patel, S. S. Tyramide-conjugated DNA barcodes enable signal amplification for multiparametric CODEX imaging. Commun. Biol. 5, 627 (2022).
Schulz, D. et al. Simultaneous multiplexed imaging of mRNA and proteins with subcellular resolution in breast cancer tissue samples by mass cytometry. Cell Syst. 6, 25–36.e5 (2018).
Acknowledgements
The authors apologize to authors whose work we have not cited owing to the focus of this Perspective article and space constraints. The authors are grateful to G. Ursini-Siegel for critical reading of this manuscript. M.B. is supported by the International Postdoc Grant from the Swedish Research Council (VR). M.J.A. is sponsored by a Quebec Research Fund (FRQS) doctorate fellowship. Work in the S.V.D.R. Laboratory is funded by the Canadian Institutes of Health Research (grant PJT-162260) and by the Canadian Cancer Society (grant 707140). The research team of C.R. is supported by grants from Swedish Cancer Association (CAN 2019/028), Swedish Research Council (VR 2021-02915), the Stockholm Cancer society and the Childhood Cancer Foundation; the laboratory of O.L. is supported by grants from the Swedish Research Council (2020-01665), the Swedish Cancer Society (19 0314), the Stockholm Cancer Society and the Wallenberg Academy Fellow’s programme.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to discussion of the content and reviewed/edited the manuscript before submission. S.V.d.R., M.B., M.J.A., O.L. and C.R. researched data for the article and wrote the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Peer review
Peer review information
Nature Reviews Cancer thanks Caroline Robert and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- G-quadruplexes
-
A type of secondary structure that can be adopted by nucleic acid sequences.
- Puromycin incorporation
-
A method that quantifies the global level of protein synthesis.
- Rocaglates
-
A family of compounds that perturb the activity of the eIF4F subunit eIF4A through a gain-of-function mechanism.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bartish, M., Abraham, M.J., Gonçalves, C. et al. The role of eIF4F-driven mRNA translation in regulating the tumour microenvironment. Nat Rev Cancer 23, 408–425 (2023). https://doi.org/10.1038/s41568-023-00567-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00567-5
This article is cited by
-
The molecular basis of translation initiation and its regulation in eukaryotes
Nature Reviews Molecular Cell Biology (2024)