Abstract
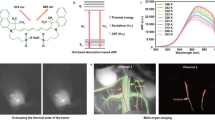
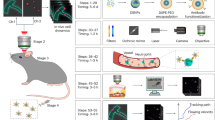
Optical imaging in the second near-infrared window (NIR-II, 1,000–1,700 nm) holds great promise for non-invasive in vivo detection. However, real-time dynamic multiplexed imaging remains challenging due to the lack of available fluorescence probes and multiplexing techniques in the ideal NIR-IIb (1,500–1,700 nm) ‘deep-tissue-transparent’ sub-window. Here we report on thulium-based cubic-phase downshifting nanoparticles (α-TmNPs) with 1,632 nm fluorescence amplification. This strategy was also validated for the fluorescence enhancement of nanoparticles doped with NIR-II Er3+ (α-ErNPs) or Ho3+ (α-HoNPs). In parallel, we developed a simultaneous dual-channel imaging system with high spatiotemporal synchronization and accuracy. The NIR-IIb α-TmNPs and α-ErNPs facilitated the non-invasive real-time dynamic multiplexed imaging of cerebrovascular vasomotion activity and the single-cell-level neutrophil behaviour in mouse subcutaneous tissue and ischaemic stroke model.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
The code that has been used for this work is available from the corresponding authors upon request.
References
Lu, L. & Zhang, F. A deep tissue optical sensing. Nat. Nanotechnol. 17, 566–568 (2022).
Huang, J. et al. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 18, 1133–1143 (2019).
Shuhendler, A. J. et al. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat. Biotechnol. 32, 373–380 (2014).
Akemann, W. et al. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat. Methods 7, 643–649 (2010).
Germain, R. N. et al. A decade of imaging cellular motility and interaction dynamics in the immune system. Science 336, 1676–1681 (2012).
Ellenbroek, S. I. J. & van Rheenen, J. Imaging hallmarks of cancer in living mice. Nat. Rev. Cancer 14, 406–418 (2014).
Harney, A. S. et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage–derived VEGFA. Cancer Discov. 5, 932–943 (2015).
Beura, L. K. et al. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 19, 173–182 (2018).
Damisah, E. C. et al. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat. Neurosci. 20, 1023–1032 (2017).
Gonzales, A. L. et al. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc. Natl Acad. Sci. USA 117, 27022–27033 (2020).
Hartmann, D. A. et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 24, 633–645 (2021).
Hofer, S. B. et al. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat. Neurosci. 14, 1045–1052 (2011).
Knopfel, T. Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 13, 687–700 (2012).
Mehlenbacher, R. D. et al. Nanomaterials for in vivo imaging of mechanical forces and electrical fields. Nat. Rev. Mater. 3, 17080 (2018).
Hontani, Y. et al. Multicolor three-photon fluorescence imaging with single-wavelength excitation deep in mouse brain. Sci. Adv. 7, eabf3531 (2021).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photon. 7, 205–209 (2013).
Liu, H. J. et al. In vivo deep-brain structural and hemodynamic multiphoton microscopy enabled by quantum dots. Nano Lett. 19, 5260–5265 (2019).
Xu, H. et al. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 10, 549–551 (2007).
Hong, G. et al. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Bruns, O. T. et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 1, 0056 (2017).
Chang, B. et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat. Biomed. Eng. 6, 629–639 (2022).
Bandi, V. G. et al. Targeted multicolor in vivo imaging over 1,000 nm enabled by nonamethine cyanines. Nat. Methods 19, 353–358 (2022).
Jia, Q. et al. Orthogonal near-infrared-II imaging enables spatially distinguishing tissues based on lanthanide-doped nanoprobes. Anal. Chem. 92, 14762–14768 (2020).
Ortgies, D. H. et al. Lifetime-encoded infrared-emitting nanoparticles for in vivo multiplexed imaging. ACS Nano 12, 4362–4368 (2018).
Fan, Y. et al. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 13, 941–946 (2018).
Zhong, Y. et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 37, 1322–1331 (2019).
Cosco, E. D. et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 12, 1123–1130 (2020).
Cosco, E. D. et al. Bright chromenylium polymethine dyes enable fast, four-color in vivo imaging with shortwave infrared detection. J. Am. Chem. Soc. 143, 6836–6846 (2021).
Fan, Y. & Zhang, F. A new generation of NIR-II probes: lanthanide-based nanocrystals for bioimaging and biosensing. Adv. Opt. Mater. 7, 1801417 (2019).
Wang, F. & Liu, X. Upconversion multicolor fine-tuning: visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J. Am. Chem. Soc. 130, 5642–5643 (2008).
Zhong, Y. et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 8, 737 (2017).
Suyver, J., Aebischer, A., García-Revilla, S., Gerner, P. & Güdel, H. Anomalous power dependence of sensitized upconversion luminescence. Phys. Rev. B 71, 125123 (2005).
Liu, Y. et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy. Nature 543, 229–233 (2017).
Shi, R. & Mudring, A. V. Phonon-mediated nonradiative relaxation in Ln3+-doped luminescent nanocrystals. ACS Mater. Lett. 4, 1882–1903 (2022).
Quintanilla, M. et al. Cubic versus hexagonal—phase, size and morphology effects on the photoluminescence quantum yield of NaGdF4:Er3+/Yb3+ upconverting nanoparticles. Nanoscale 14, 1492–1504 (2022).
Shen, Z. et al. An artery-specific fluorescent dye for studying neurovascular coupling. Nat. Methods 9, 273–276 (2012).
Hill, R. A. et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110 (2015).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Kreisel, D. et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl Acad. Sci. USA 107, 18073–18078 (2010).
Stark, K. et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat. Immunol. 14, 41–51 (2013).
Ng, L. G. et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Invest. Dermatol. 131, 2058–2068 (2011).
Lim, K. et al. In situ neutrophil efferocytosis shapes T cell immunity to influenza infection. Nat. Immunol. 21, 1046–1057 (2020).
Justicia, C. et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J. Cereb. Blood Flow. Metab. 23, 1430–1440 (2003).
Schmid, M. C. et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 9, 5379 (2018).
Li, M. et al. Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat. Commun. 11, 1126 (2020).
LaFleur, M. W. et al. PTPN2 regulates the generation of exhausted CD8+ T cell subpopulations and restrains tumor immunity. Nat. Immunol. 20, 1335–1347 (2019).
Hyun, Y. M. et al. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J. Exp. Med. 209, 1349–1362 (2012).
Lim, K. et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 349, aaa4352 (2015).
Hong, G. et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 5, 4206 (2014).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC; 22088101, 21725502, 51961145403, 21904023, 11974097, 22161160320, 32171377), New Cornerstone Science Foundation through the XPLORER PRIZE and the Research Program of Science and Technology Commission of Shanghai Municipality (20JC1411700, 20490710600, 20S31903700, 21QA1406700, 22JC1400400).
Author information
Authors and Affiliations
Contributions
F.Z. and Y.F. conceived and designed the experiments. Y.Y. synthesized the nanoparticles. Y.Y. and P.P. conducted the NIR-II dual-channel synchronous imaging in vitro. Y.C. and Y.Y. conducted the imaging of the synchronous dynamic tracking of cerebrovascular vasomotion and neutrophil behaviours in mouse subcutaneous tissue and ischaemic stroke model. S.W., H.Z. and Y.Y. built the NIR-IIb spatiotemporal synchronized dual-channel NIR-IIb imaging system. Y.Y., F.Z. and Y.F. wrote the paper. F.Z., Y.F., Y.Y., Y.C., S.W., B.-Z.Q. and H.Z analysed the results, figures and supplementary information. F.Z., Y.F., Y.Y., H.Z. and D.Z. discussed the mechanism of Tm3+-based cubic-phase core–shell-structured nanoparticles. D.Z. and F.Z. also contributed to the discussions about the dual-channel NIR-IIb imaging system. All the authors contributed to discussing and editing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Zhihong Liu, Riccardo Marin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of different activators based core/multi-shell-shell nanoparticles with different crystal phases.
a, TEM images and corresponding sizes of cubic-phase (α) and hexagnal-phase (β) NaYF4:Yb/A (A = Tm, Er and Ho) core and final core-shell-shell nanocrystals with same components. Scale bar, 20 nm. To eliminate the effect of nanoparticle size on fluorescence intensity comparision, α- and β-NaYF4:Yb/A@NaYbF4@NaYF4 (A = Tm, Er and Ho) were synthesized with similar core diameter followed by the identical epitaxial growth of outer shells. b, Fluorescence emission spectra of TmNPs, ErNPs and HoNPs with α- and β-phase.
Extended Data Fig. 2 The effect of MPR process on the luminescence transitions in α-TmNPs.
a, b, Luminescence emission spectra (a) and corresponding intensities variation (b) in visible and NIR regions of α-TmNPs with increasing Tm3+ content under 980 nm laser excitation. c, Proposed MPR mediated cross-relaxation between Tm3+. d, Lifetime of α-NaYF4:Tm(x)@NaYbF4@NaYF4 at 1632 nm with varying Tm3+ content. Inset shows the lifetime changes versus Tm3+ content.
Extended Data Fig. 3 Characterization of the backward-forward energy transfer (BFET) process.
a, Simplified energy level diagram shows backward energy transfer (BET) and forward energy transfer (FET) under 808 nm excitation (absorbed by only Tm3+). Yb3+ trapped the pumped energy from energy level 3H5 of Tm3+and transfer it to lower energy level 3F4 of Tm3+. b–d, Luminescence decay curves measured at (b)1632 nm, (c) 1475 nm and (d) 800 nm from Tm3+ with increasing Yb3+ content in core. e, Luminescence emission spectra and fluorescence intensities at 980 nm emission (inset) for α-TmNPs with increasing Yb3+ content in core(e). f, Decay curves of Yb3+ (2F5/2 → 2F7/2) with increasing Yb3+ content in core. g, Normalized luminescence lifetime in (b), (c), (d), (f).
Extended Data Fig. 4 Characterization of core-shell-shell α-HoNPs and α-ErNPs with different Yb3+ content.
a, Simplified energy level diagrams of α-HoNPs and α-ErNPs show backward energy transfer (BET) and forward energy transfer (FET) under 980 nm excitation or 655 nm excitaion. b, c, Luminescence steady-state emission spectra and intensities (insets) for α-HoNPs with increasing Yb3+ content under (b) 980 nm and (c) 655 nm excitation. Insets show the luminescence intensity. d, e, Luminescence steady-state emission spectra and intensities for α-ErNPs with increasing Yb3+ content under (b) 980 nm and (c) 655 nm excitation.
Extended Data Fig. 5 Characterization of image resolution covered with biological tissues in varied NIR-II sub-regions.
a, Typical SEM image of the PS beasds (~ 10 μm) used as the substrates for cubic-phase Tm3+-, Er3+- and Ho3+-based nanoparticles encapulation. Scale bar, 20 μm. b, Fabrication procedures for polystyrene (PS) beads tagged with single component (α-TmNPs, α-ErNPs or α-HoNPs). c, Schematic of in vitro imaging experiments using the DSNPs-tagged beads imaging that covered with biological tissues. The inset shows physical picture of mouse skull with a thickness of ~1 mm. d, e Fluorescence images of the three types of PS beads tagged with α-TmNPs (Tm-Ch, 1600–1700 nm), α-ErNPs (Er-Ch, 1500–1600 nm) and α-HoNPs (Ho-Ch, 1100–1200 nm), respectively, in the absence and presence of a covered slice of (d) mouse skin or (e) skull and corresponding image resolutions. The imaging resolutions were quantified by full width at half-maximum (FWHM) of a PS bead along the white-dased lines. Exposure time: 300 ms. Scale bar, 50 μm.
Extended Data Fig. 6 Real-time dynamic NIR-IIb multiplexed imaging of Tm/Er tagged PS beads.
a, Fabrication procedures for polystyrene (PS) beads tagged with both α-TmNPs and α-ErNPs (Tm/Er tagged beads). b, NIR-IIb fluorescence images of Tm/Er-tagged PS beads in Tm-Ch or Er-Ch. The overlapping image shows well co-localization of the two channels. Scale bar, 50 μm. c, A photograph of the simultaneous dual-channel NIR-IIb fluorescence imaging system equipped with a microfluidic system. Tm/Er- tagged PS-beads were dispersed in cetane, followed by being pumped into the flow channel of the glass chip. d, Real-time multiplexed images of Tm/Er-tagged PS beads flowing in the channel of the glass chip at a fixed perfusion rate (100 mL/h). Elongated outline of the PS beads along the direction of fluid flowing was attributed to motion-blur under exposure time of ~ 300 ms. Scale bar, 50 μm. e, Statistical results of the average velocities in the Er-Ch and Tm-Ch. Quantitative results showed mean velocities of PS beads in a range of 4437.7–4657.0 μm/min in Tm-Ch and 4524.3–4675.4 μm/min in Er-Ch, with minor deviation between two imaging channels (less than 2% as shown in the right side of the figure). The data are shown as the mean ± s.d. (n = 17–18). A boxplot with minimum, maximum and median is shown. f, g, Pearson’s correlation (Pr value) analysis of (f) velocity and (g) location of the six tracked PS beads between Er- and Tm-Ch in a, indicating a high spatiotemporal synchronicity.
Extended Data Fig. 7 Whole body blood vessel imaging of mouse in Tm-, Er- and Ho-Ch.
a,c, NIR-II images of mouse blood vessels in (a) supine and (c) prone posistion with intravenous injection of aqueous α-TmNPs, α-ErNPs and α-HoNPs, respectively. b, d, Correponding signal-to-background ratios (SBRs) of mouse blood vessels in (b) belly and (d) prone posistion by plotting the cross-sectional intensity profiles as shown in the dased line in a and c. Scale bar, 1 cm. Exposure time: 300 ms. Whole-body blood imaging in NIR-IIb window presents SBRs of 3.0–12.5 (Tm-Ch and Er-Ch), which were ~1.0–4.3 fold higher over that in short wavelength NIR region (2.9–4.3) (Ho-Ch).
Extended Data Fig. 8 Noninvasive monitoring the specific labelling of elasitc fibres of arteries by α-Er@Af633 nanoprobes.
a, Time-line of monitoring of the α-Er@Af633 labeled elastic fibres. Free α-ErNPs without conjugation of Alexa Fluor 633 dyes were also adopted with identical experimental procedures as a control group. b, c, Real-time tracking metabolic processes of (b) α-Er@Af633 or (c) free α-ErNPs in vessel blood. Scale bar, 20 μm. Blood vessels with similar lumen diameters were monitored for comparing the metabolic behaviors of α-Er@Af633 or free α-ErNPs. d, e, Quantitative analysis of metabolic behaviors of (d) α-Er@Af633 and (e) free α-ErNPs in blood vessels by plotting the signal intensity along the whited dashed line in (b) and (c). Decreased fluorescence intensity of the vessel lumen was observed with injection of α-Er@Af 633 over time. f, Corresponding SBRs of the artery walls with injection of α-Er@Af 633 (from 3.0 to 4.4) or free α-ErNPs (from 2.8 to 1.3).
Extended Data Fig. 9 Real-time dynamic multiplexed imaging of neutrophil behaviors in acute inflammation.
a, Multiplexed dynamic tracking of a single neutrophil (blue-dashed circle) migrating towards a small neutrophil cluster (white arrow) in the inflamed site in mice ear. The vascular profile was depicted by white dotted line. Scale bar, 20 μm. b, Migratory paths of the single neutrophil (presented as x-y projections). The calculated meandering index (MI) value of ~ 0.81 (>0.8) was commonly associated with chemotaxis, indicating its potential to be activated in inflammatory microenvironment. c, Real-time imaging of neutrophil extravasation (purple-dashed circle), which is a typical characteristic of activated state. Scale bar, 50 μm.
Extended Data Fig. 10 In vivo NIR-IIb imaging of neutrophil recruitment in stroke mice through intact skull using α-Tm-aLy6G.
Imaging was performed independently on n = 4 biological replicates for each group with similar results. Neutrophils were always considered as short-lived cells with a circulation half-life of approximately 1.5 h in healthy mice, however, their circulating period can be increased by several folds if inflammation occurred. NIR-IIb signal from α-Tm-aLy6G was clearly observed in the injured left cerebral hemisphere and increased over time (also shown in Fig. 5g in the manuscript), with the brightest intensity obtained at the 24 h p.i, which indicates inflammation responsive neutrophil recruitment.
Supplementary information
Supplementary Information
Supplementary Figs. 1–44, Tables 1–5, Imaging data analyses and References.
Supplementary Video 1
Synchronous multiplexed tracking of Tm/Er-tagged beads.
Supplementary Video 2
Synchronous multiplexed dynamic tracking of artery vasoconstriction.
Supplementary Video 3
Dynamic tracking of spontaneous vasomotion of cerebral network.
Supplementary Video 4
Neutrophil dynamics in inflamed (LPS+) and normal (Ctrl) sites.
Supplementary Video 5
Multiplexed dynamic tracking of neutrophil behaviors in acute inflammation.
Supplementary Video 6
Behavioural symptom of stroke mice.
Supplementary Video 7
Synchronous multiplexed dynamic tracking of activated neutrophils.
Supplementary Data
Statistical source data for Supplementary Figs. 28, 31, 34, 42 and 43.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Source data.
Source Data Extended Data Fig. 2
Source data.
Source Data Extended Data Fig. 3
Source data.
Source Data Extended Data Fig. 4
Source data.
Source Data Extended Data Fig. 5
Source data.
Source Data Extended Data Fig. 6
Source data.
Source Data Extended Data Fig. 7
Source data.
Source Data Extended Data Fig. 8
Source data.
Source Data Extended Data Fig. 9
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Chen, Y., Pei, P. et al. Fluorescence-amplified nanocrystals in the second near-infrared window for in vivo real-time dynamic multiplexed imaging. Nat. Nanotechnol. 18, 1195–1204 (2023). https://doi.org/10.1038/s41565-023-01422-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01422-2
This article is cited by
-
Near-infrared II fluorescence imaging
Nature Reviews Methods Primers (2024)
-
In vivo NIR-II fluorescence imaging for biology and medicine
Nature Photonics (2024)
-
Noninvasive in vivo microscopy of single neutrophils in the mouse brain via NIR-II fluorescent nanomaterials
Nature Protocols (2024)