Abstract
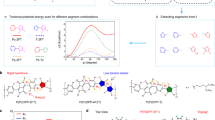
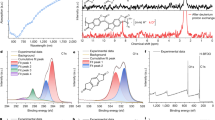
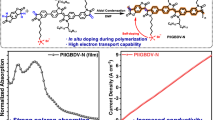
π-Conjugated polymers (CPs) have broad applications in high-performance optoelectronics, energy storage, sensors and biomedicine. However, developing green and efficient methods to precisely synthesize alternating CP structures on a large scale remains challenging and critical for their industrialization. Here a room-temperature, scalable and homogeneous Suzuki–Miyaura-type polymerization reaction is developed with broad generality validated for 24 CPs including donor–donor, donor–acceptor and acceptor–acceptor connectivities, yielding device-quality polymers with high molecular masses. Furthermore, the polymerization protocol significantly reduces homocoupling structural defects, yielding more structurally regular and higher-performance electronic materials and optoelectronic devices than conventional thermally activated polymerizations. Experimental and theoretical studies reveal that a borate transmetalation process plays a key role in suppressing protodeboronation, which is critical for large-scale structural regularity. Thus, these results provide a general polymerization tool for the scalable production of device-quality CPs with alternating structural regularity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study are available in the Article and its Supplementary Information. Synthesis procedures and characterization for all the new compounds, computational studies and all copies of NMR spectra and gel permeation chromatography traces are available in the Supplementary Information. Source data are provided with this paper.
Change history
05 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41563-024-01824-0
References
Fratini, S., Nikolka, M., Salleo, A., Schweicher, G. & Sirringhaus, H. Charge transport in high-mobility conjugated polymers and molecular semiconductors. Nat. Mater. 19, 491–502 (2020).
Wang, J., Xue, P., Jiang, Y., Huo, Y. & Zhan, X. The principles, design and applications of fused-ring electron acceptors. Nat. Rev. Chem. 6, 614–634 (2022).
Lopez, J., Mackanic, D. G., Cui, Y. & Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 4, 312–330 (2019).
Dou, L., Liu, Y., Hong, Z., Li, G. & Yang, Y. Low-bandgap near-IR conjugated polymers/molecules for organic electronics. Chem. Rev. 115, 12633–12665 (2015).
Jiang, Y. & Pu, K. Multimodal biophotonics of semiconducting polymer nanoparticles. Acc. Chem. Res. 51, 1840–1849 (2018).
Hendriks, K. H. et al. Homocoupling defects in diketopyrrolopyrrole-based copolymers and their effect on photovoltaic performance. J. Am. Chem. Soc. 136, 11128–11133 (2014).
Streiter, M. et al. Homocoupling defects in a conjugated polymer limit exciton diffusion. Adv. Funct. Mater. 29, 1903936 (2019).
Ni, Z. et al. Mesopolymer synthesis by ligand-modulated direct arylation polycondensation towards n-type and ambipolar conjugated systems. Nat. Chem. 11, 271–277 (2019).
Ji, X. et al. Pauli paramagnetism of stable analogues of pernigraniline salt featuring ladder-type constitution. J. Am. Chem. Soc. 142, 641–648 (2020).
Lee, S. M., Park, K. H., Jung, S., Park, H. & Yang, C. Stepwise heating in Stille polycondensation toward no batch-to-batch variations in polymer solar cell performance. Nat. Commun. 9, 1867 (2018).
Wang, Z. et al. Unraveling the molar mass dependence of shearing-induced aggregation structure of a high-mobility polymer semiconductor. Adv. Mater. 34, 2108255 (2022).
Vangerven, T. et al. Elucidating batch-to-batch variation caused by homocoupled side products in solution-processable organic solar cells. Chem. Mater. 28, 9088–9098 (2016).
Yang, J., Zhao, Z., Wang, S., Guo, Y. & Liu, Y. Insight into high-performance conjugated polymers for organic field-effect transistors. Chem 4, 2748–2785 (2018).
Burke, D. J. & Lipomi, D. J. Green chemistry for organic solar cells. Energy Environ. Sci. 6, 2053–2066 (2013).
Sakamoto, J., Rehahn, M., Wegner, G. & Schlüter, A. D. Suzuki polycondensation: polyarylenes à la carte. Macromol. Rapid Commun. 30, 653–687 (2009).
Zhang, H.-H., Xing, C.-H. & Hu, Q.-S. Controlled Pd(0)/t-Bu3P-catalyzed Suzuki cross-coupling polymerization of AB-type monomers with PhPd(t-Bu3P)I or Pd2(dba)3/t-Bu3P/ArI as the initiator. J. Am. Chem. Soc. 134, 13156–13159 (2012).
Peng, W., Dong, J., Li, H.-B., Chow, C. & Hu, Q.-S. Room temperature Suzuki cross-coupling polymerizations of aryl dihalides and aryldiboronic acid/acid esters with t-Bu3P-coordinated 2-phenylaniline-based palladacycle complex as the precatalyst. J. Polym. Sci. A: Polym. Chem. 57, 1606–1611 (2019).
Carsten, B., He, F., Son, H. J., Xu, T. & Yu, L. Stille polycondensation for synthesis of functional materials. Chem. Rev. 111, 1493–1528 (2011).
Lo, C. K., Wolfe, R. M. W. & Reynolds, J. R. From monomer to conjugated polymer: a perspective on best practices for synthesis. Chem. Mater. 33, 4842–4852 (2021).
Yokozawa, T. & Ohta, Y. Transformation of step-growth polymerization into living chain-growth polymerization. Chem. Rev. 116, 1950–1968 (2016).
Pouliot, J.-R., Grenier, F., Blaskovits, J. T., Beaupré, S. & Leclerc, M. Direct (hetero)arylation polymerization: simplicity for conjugated polymer synthesis. Chem. Rev. 116, 14225–14274 (2016).
Cordovilla, C., Bartolomé, C., Martínez-Ilarduya, J. M. & Espinet, P. The Stille reaction, 38 years later. ACS Catal. 5, 3040–3053 (2015).
Bura, T. et al. Direct heteroarylation polymerization: guidelines for defect-free conjugated polymers. Chem. Sci. 8, 3913–3925 (2017).
Blaskovits, J. T., Johnson, P. A. & Leclerc, M. Mechanistic origin of β-defect formation in thiophene-based polymers prepared by direct (hetero)arylation. Macromolecules 51, 8100–8113 (2018).
Suzuki, A. Cross-coupling reactions of organoboranes: an easy way to construct C–C bonds (Nobel lecture). Angew. Chem. Int. Ed. 50, 6722–6737 (2011).
van Asselt, R. & Elsevier, C. J. On the mechanism of formation of homocoupled products in the carbon-carbon cross-coupling reaction catalyzed by palladium complexes containing rigid bidentate nitrogen ligands: evidence for the exchange of organic groups between palladium and the transmetalating reagent. Organometallics 13, 1972–1980 (1994).
Adamo, C., Amatore, C., Ciofini, I., Jutand, A. & Lakmini, H. Mechanism of the palladium-catalyzed homocoupling of arylboronic acids: key involvement of a palladium peroxo complex. J. Am. Chem. Soc. 128, 6829–6836 (2006).
Rudenko, A. E. & Thompson, B. C. Optimization of direct arylation polymerization (DArP) through the identification and control of defects in polymer structure. J. Polym. Sci. A: Polym. Chem. 53, 135–147 (2015).
Li, Z. et al. Efficient room temperature catalytic synthesis of alternating conjugated copolymers via C-S bond activation. Nat. Commun. 13, 144 (2022).
Cox, P. A. et al. Base-catalyzed aryl-B(OH)2 protodeboronation revisited: from concerted proton transfer to liberation of a transient aryl anion. J. Am. Chem. Soc. 139, 13156–13165 (2017).
Malapit, C. A., Bour, J. R., Brigham, C. E. & Sanford, M. S. Base-free nickel-catalysed decarbonylative Suzuki–Miyaura coupling of acid fluorides. Nature 563, 100–104 (2018).
Jayakannan, M., van Dongen, J. L. J. & Janssen, R. A. J. Mechanistic aspects of the Suzuki polycondensation of thiophenebisboronic derivatives and diiodobenzenes analyzed by MALDI–TOF mass spectrometry. Macromolecules 34, 5386–5393 (2001).
Gibson, G. L., McCormick, T. M. & Seferos, D. S. Atomistic band gap engineering in donor–acceptor polymers. J. Am. Chem. Soc. 134, 539–547 (2012).
Lee, J.-K. et al. High-performance electron-transporting polymers derived from a heteroaryl bis(trifluoroborate). J. Am. Chem. Soc. 133, 9949–9951 (2011).
Carrillo, J. A., Turner, M. L. & Ingleson, M. J. A general protocol for the polycondensation of thienyl N-methyliminodiacetic acid boronate esters to form high molecular weight copolymers. J. Am. Chem. Soc. 138, 13361–13368 (2016).
Hazari, N., Melvin, P. R. & Beromi, M. M. Well-defined nickel and palladium precatalysts for cross-coupling. Nat. Rev. Chem. 1, 0025 (2017).
Bürglová, K. & Hlaváč, J. Application of trimethylsilanolate alkali salts in organic synthesis. Synthesis 50, 1199–1208 (2018).
Billingsley, K. L. & Buchwald, S. L. A general and efficient method for the Suzuki–Miyaura coupling of 2-pyridyl nucleophiles. Angew. Chem. Int. Ed. 47, 4695–4698 (2008).
Makida, Y., Marelli, E., Slawin, A. M. Z. & Nolan, S. P. Nickel-catalysed carboxylation of organoboronates. Chem. Commun. 50, 8010–8013 (2014).
Yoshida, H. et al. Direct Suzuki–Miyaura coupling with naphthalene-1,8-diaminato (dan)-substituted organoborons. ACS Catal. 10, 346–351 (2020).
Delaney, C. P., Kassel, V. M. & Denmark, S. E. Potassium trimethylsilanolate enables rapid, homogeneous Suzuki–Miyaura cross-coupling of boronic esters. ACS Catal. 10, 73–80 (2020).
Niwa, T. et al. Lewis acid-mediated Suzuki–Miyaura cross-coupling reaction. Nat. Catal. 4, 1080–1088 (2021).
Carothers, W. H. Polymerization. Chem. Rev. 8, 353–426 (1931).
He, Z.-T., Li, H., Haydl, A. M., Whiteker, G. T. & Hartwig, J. F. Trimethylphosphate as a methylating agent for cross coupling: a slow-release mechanism for the methylation of arylboronic esters. J. Am. Chem. Soc. 140, 17197–17202 (2018).
Wang, Y., Hasegawa, T., Matsumoto, H. & Michinobu, T. Significant difference in semiconducting properties of isomeric all-acceptor polymers synthesized via direct arylation polycondensation. Angew. Chem. Int. Ed. 58, 11893–11902 (2019).
Li, Y. et al. Annealing-free high-mobility diketopyrrolopyrrole–quaterthiophene copolymer for solution-processed organic thin film transistors. J. Am. Chem. Soc. 133, 2198–2204 (2011).
Sun, B., Hong, W., Aziz, H., Abukhdeir, N. M. & Li, Y. Dramatically enhanced molecular ordering and charge transport of a DPP-based polymer assisted by oligomers through antiplasticization. J. Mater. Chem. C 1, 4423–4426 (2013).
Spano, F. C. & Silva, C. H- and J-aggregate behavior in polymeric semiconductors. Annu. Rev. Phys. Chem. 65, 477–500 (2014).
Lombeck, F. et al. On the effect of prevalent carbazole homocoupling defects on the photovoltaic performance of PCDTBT:PC71BM solar cells. Adv. Energy Mater. 6, 1601232 (2016).
Acknowledgements
We acknowledge financial support from the NSFC (51925306 (H.H.), 52222309 (Q.S.) and 52173187 (Q.S.)), National Key R&D Program of China (2018FYA 0305800 (H.H.)), Key Research Program of the Chinese Academy of Sciences (XDPB08-2 (H.H.)), China Postdoctoral Science Foundation (2021M703158 (H.X.)) and Fundamental Research Funds for the Central University. T.J.M. thanks the National Science Foundation Materials Research Science and Engineering Center at Northwestern University (DMR-2308691) and the Air Force Office of Scientific Research (FA9550-22-1-0423) for support. We thank X. Hao and J. Qiao from Shandong University for the exciton diffusion length measurements.
Author information
Authors and Affiliations
Contributions
H.H. directed the investigations and provided overall supervision. H.X. designed the experiments. H.X., W.X., X.Z. and Q.S. performed the synthesis experiments. Q.L. and D.Z. performed the device fabrications. Y. Lu, S.W. and Z.W. performed the DFT calculations. Y. Li and Y. Lin performed the trap density measurements. S.W., C.L. and X.Z. performed the atomic force microscopy and GIWAXS measurements. H.X., Q.S., T.J.M. and H.H. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–22, discussion and Tables 1–9.
Source data
Source Data Fig. 4
Source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, H., Lin, Q., Lu, Y. et al. General room-temperature Suzuki–Miyaura polymerization for organic electronics. Nat. Mater. (2024). https://doi.org/10.1038/s41563-023-01794-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-023-01794-9