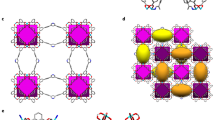
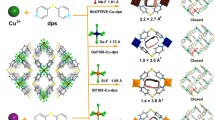
Abstract
Carbon monoxide (CO) separation relies on chemical adsorption but suffers from the difficulty of desorption and instability of open metal sites against O2, H2O and so on. Here we demonstrate quasi-open metal sites with hidden or shielded coordination sites as a promising solution. Possessing the trigonal coordination geometry (sp2), Cu(i) ions in porous frameworks show weak physical adsorption for non-target guests. Rational regulation of framework flexibility enables geometry transformation to tetrahedral geometry (sp3), generating a fourth coordination site for the chemical adsorption of CO. Quantitative breakthrough experiments at ambient conditions show CO uptakes up to 4.1 mmol g−1 and CO selectivity up to 347 against CO2, CH4, O2, N2 and H2. The adsorbents can be completely regenerated at 333–373 K to recover CO with a purity of >99.99%, and the separation performances are stable in high-concentration O2 and H2O. Although CO leakage concentration generally follows the structural transition pressure, large amounts (>3 mmol g−1) of ultrahigh-purity (99.9999999%, 9N; CO concentration < 1 part per billion) gases can be produced in a single adsorption process, demonstrating the usefulness of this approach for separation applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this Article and its Supplementary Information. Additional data are available from the corresponding author upon request.
References
Kerry, F. G. Industrial Gas Handbook: Gas Separation and Purification (CRC Press, 2007).
Reed, D. A. et al. A spin transition mechanism for cooperative adsorption in metal-organic frameworks. Nature 550, 96–100 (2017).
Sato, H. et al. Self-accelerating CO sorption in a soft nanoporous crystal. Science 343, 167–170 (2014).
Wang, H., Lustig, W. P. & Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal-organic frameworks. Chem. Soc. Rev. 47, 4729–4756 (2018).
Dutta, N. N. & Patil, G. S. Developments in CO separation. Gas. Sep. Purif. 9, 277–283 (1995).
Islamoglu, T. et al. Metal-organic frameworks against toxic chemicals. Chem. Rev. 120, 8130–8160 (2020).
DeCoste, J. B. & Peterson, G. W. Metal-organic frameworks for air purification of toxic chemicals. Chem. Rev. 114, 5695–5727 (2014).
Evans, A., Luebke, R. & Petit, C. The use of metal-organic frameworks for CO purification. J. Mater. Chem. A 6, 10570–10594 (2018).
Frohning C. D., Kohlpaintner C. W. & Bohnen H. W. Carbon Monoxide and Synthesis Gas Chemistry (Wiley-VCH, 2008).
Van Rooij, G. J. et al. Taming microwave plasma to beat thermodynamics in CO2 dissociation. Faraday Discuss. 183, 233–248 (2015).
Cheng, X. et al. A review of PEM hydrogen fuel cell contamination: impacts, mechanisms, and mitigation. J. Power Sources 165, 739–756 (2007).
Hydrogen fuel quality—product specification (ISO 14687, 2019).
King, C. J. Handbook of Separation Process Technology (John Wiley & Sons, 1987).
Li, G. Q. & Govind, R. Separation of oxygen from air using coordination complexes: a review. Ind. Eng. Chem. Res. 33, 755–783 (1994).
Morris, R. E. & Wheatley, P. S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 47, 4966–4981 (2008).
Li, J.-R., Sculley, J. & Zhou, H.-C. Metal-organic frameworks for separations. Chem. Rev. 112, 869–932 (2012).
Cadiau, A. et al. Hydrolytically stable fluorinated metal-organic frameworks for energy-efficient dehydration. Science 356, 731–735 (2017).
Yoon, J. W. et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites. Nat. Mater. 16, 526–531 (2017).
Smith, G. L. et al. Reversible coordinative binding and separation of sulfur dioxide in a robust metal-organic framework with open copper sites. Nat. Mater. 18, 1358–1365 (2019).
Schmieder, P., Denysenko, D., Grzywa, M., Magdysyuk, O. & Volkmer, D. A structurally flexible triazolate-based metal-organic framework featuring coordinatively unsaturated copper(i) sites. Dalton Trans. 45, 13853–13862 (2016).
Peng, J. et al. A supported Cu(i)@MIL-100(Fe) adsorbent with high CO adsorption capacity and CO/N2 selectivity. Chem. Eng. J. 270, 282–289 (2015).
Gao, F., Wang, Y. & Wang, S. Selective adsorption of CO on CuCl/Y adsorbent prepared using CuCl2 as precursor: equilibrium and thermodynamics. Chem. Eng. J. 290, 418–427 (2016).
Evans, A. D. et al. Screening metal-organic frameworks for dynamic CO/N2 separation using complementary adsorption measurement techniques. Ind. Eng. Chem. Res. 58, 18336–18344 (2019).
Reed, D. A. et al. Reversible CO scavenging via adsorbate-dependent spin state transitions in an iron(ii)-triazolate metal-organic framework. J. Am. Chem. Soc. 138, 5594–5602 (2016).
Bloch, E. D. et al. Reversible CO binding enables tunable CO/H2 and CO/N2 separations in metal-organic frameworks with exposed divalent metal cations. J. Am. Chem. Soc. 136, 10752–10761 (2014).
Denysenko, D., Grzywa, M., Jelic, J., Reuter, K. & Volkmer, D. Scorpionate-type coordination in MFU-4l metal-organic frameworks: small-molecule binding and activation upon the thermally activated formation of open metal sites. Angew. Chem. Int. Ed. 53, 5832–5836 (2014).
Bloch, E. D. et al. Selective binding of O2 over N2 in a redox-active metal-organic framework with open iron(ii) coordination sites. J. Am. Chem. Soc. 133, 14814–14822 (2011).
Wang, J. et al. Optimizing pore space for flexible-robust metal-organic framework to boost trace acetylene removal. J. Am. Chem. Soc. 142, 9744–9751 (2020).
Horike, S., Inubushi, Y., Hori, T., Fukushima, T. & Kitagawa, S. A solid solution approach to 2D coordination polymers for CH4/CO2 and CH4/C2H6 gas separation: equilibrium and kinetic studies. Chem. Sci. 3, 116–120 (2012).
Zhang, X.-W., Zhou, D.-D. & Zhang, J.-P. Tuning the gating energy barrier of metal-organic framework for molecular sieving. Chem 7, 1006–1019 (2021).
Zhang, J.-P. & Chen, X.-M. Exceptional framework flexibility and sorption behavior of a multifunctional porous cuprous triazolate framework. J. Am. Chem. Soc. 130, 6010–6017 (2008).
Zhang, J.-P. & Chen, X.-M. Optimized acetylene/carbon dioxide sorption in a dynamic porous crystal. J. Am. Chem. Soc. 131, 5516–5521 (2009).
Liu, S.-Y. et al. Flexible, luminescent metal-organic frameworks showing synergistic solid-solution effects on porosity and sensitivity. Angew. Chem. Int. Ed. 55, 16021–16025 (2016).
Zhuo, L.-L. et al. Flexible cuprous triazolate frameworks as highly stable and efficient electrocatalysts for CO2 reduction with tunable C2H4/CH4 selectivity. Angew. Chem. Int. Ed. 61, e202204967 (2022).
Wang, C. et al. A partially fluorinated ligand for two super-hydrophobic porous coordination polymers with classic structures and increased porosities. Natl Sci. Rev. 8, nwaa094 (2021).
Krause, S. et al. The impact of crystal size and temperature on the adsorption-induced flexibility of the Zr-based metal–organic framework DUT-98. Beilstein J. Nanotechnol. 10, 1737–1744 (2019).
Numaguchi, R., Tanaka, H., Watanabe, S. & Miyahara, M. T. Simulation study for adsorption-induced structural transition in stacked-layer porous coordination polymers: equilibrium and hysteretic adsorption behaviors. J. Chem. Phys. 138, 054708 (2013).
Lopes, F. V. S. et al. Adsorption of H2, CO2, CH4, CO, N2 and H2O in activated carbon and zeolite for hydrogen production. Sep. Sci. Technol. 44, 1045–1073 (2009).
Chen, K.-J. et al. Efficient CO2 removal for ultra-pure CO production by two hybrid ultramicroporous materials. Angew. Chem. Int. Ed. 57, 3332–3336 (2018).
Evans, J. D., Bon, V., Senkovska, I., Lee, H.-C. & Kaskel, S. Four-dimensional metal-organic frameworks. Nat. Commun. 11, 2690 (2020).
Zhou, D.-D. & Zhang, J.-P. On the role of flexibility for adsorptive separation. Acc. Chem. Res. 55, 2966–2977 (2022).
Krause, S., Hosono, N. & Kitagawa, S. Chemistry of soft porous crystals: structural dynamics and gas adsorption properties. Angew. Chem. Int. Ed. 59, 15325–15341 (2020).
Wayner, D. D. M. & Arnold, D. R. Substituent effects on benzylic radical hydrogen hyperfine coupling constants. Part 4. The effect of branching of the alkyl substituent. Can. J. Chem. 63, 2378–2383 (1985).
Van Wüllen, C. Molecular structure and binding energies of monosubstituted hexacarbonyls of chromium, molybdenum, and tungsten: relativistic density functional study. J. Comput. Chem. 18, 1985–1992 (1997).
Coudert, F.-X., Jeffroy, M., Fuchs, A. H., Boutin, A. & Mellot-Draznieks, C. Thermodynamics of guest-induced structural transitions in hybrid organic-inorganic frameworks. J. Am. Chem. Soc. 130, 14294–14302 (2008).
Weiss, J. N. The Hill equation revisited: uses and misuses. FASEB J. 11, 835–841 (1997).
Van Albada, G. A., De Graaff, R. A. G., Haasnoot, J. G. & Reedijk, J. Synthesis, spectroscopic characterization, and magneticproperties of unusual 3,5-dialkyl-1,2,4-triazole compounds containing N-bridging isothiocyanato ligands. X-ray structure of trinuclear bis[(μ-thiocyanato-N)bis(μ-3,5-diethyl-1,2,4-triazole-N1,N2)bis(thiocyanato-N)(3,5-diethyl-1,2,4-triazole-N1)nickel(ii)-N,N1,N1']nickel(ii) dihydrate. Inorg. Chem. 23, 1404–1408 (1984).
Xue, H., Twamley, B. & Shreeve, J. M. The first 1-alkyl-3-perfluoroalkyl-4,5-dimethyl-1,2,4-triazolium salts. J. Org. Chem. 69, 1397–1400 (2004).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Rehr, J. J., Kas, J. J., Vila, F. D., Prange, M. P. & Jorissen, K. Parameter-free calculations of X-ray spectra with FEFF9. Phys. Chem. Chem. Phys. 12, 5503–5513 (2010).
Moosavi, S. M. et al. A data-science approach to predict the heat capacity of nanoporous materials. Nat. Mater. 21, 1419–1425 (2022).
Bernardes, C. E. S., Santos, L. M. N. B. F. & da Piedade, M. E. M. A new calorimetric system to measure heat capacities of solids by the drop method. Meas. Sci. Technol. 17, 1405 (2006).
Huang, N.-Y. et al. Direct synthesis of an aliphatic amine functionalized metal-organic framework for efficient CO2 removal and CH4 purification. CrystEngComm 20, 5969–5975 (2018).
Ye, Z.-M. et al. A hydrogen-bonded yet hydrophobic porous molecular crystal for molecular-sieving-like separation of butane and isobutane. Angew. Chem. Int. Ed. 59, 23322–23328 (2020).
Kühne, T. D. et al. CP2K: an electronic structure and molecular dynamics software package—Quickstep: efficient and accurate electronic structure calculations. J. Chem. Phys. 152, 194103 (2020).
Xiao, M. & Lu, T. Generalized charge decomposition analysis (GCDA) method. J. Adv. Phys. Chem. 4, 111–124 (2015).
Frisch, M. J. et al. Gaussian09 (Gaussian, 2009).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Acknowledgements
J.-P.Z. acknowledges support by the National Natural Science Foundation of China (22231012, 22090061 and 21821003) and the XPLORER PRIZE. We thank J.X. Jiang for help with the infrared spectroscopy experiments and Z.F. Ke for help with the computational simulation.
Author information
Authors and Affiliations
Contributions
J.-P.Z. conceived and designed the research. X.-W.Z., C.W. and Z.-W.M. performed the syntheses and measurements. C.W., X.-W.Z., X.-X.C. and W.-X.Z. carried out the structural analyses. X.-W.Z. carried out the breakthrough experiments and theoretical calculations. C.W. collected the infrared spectra. X.-W.Z., C.W. and J.-P.Z. wrote the manuscript, and all authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Satoshi Horike and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–116 and Tables 1–22.
Supplementary Data
Computational models.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, XW., Wang, C., Mo, ZW. et al. Quasi-open Cu(i) sites for efficient CO separation with high O2/H2O tolerance. Nat. Mater. 23, 116–123 (2024). https://doi.org/10.1038/s41563-023-01729-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01729-4
This article is cited by
-
Engineering porous crystals to do different things
Nature Materials (2024)