Abstract
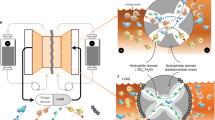
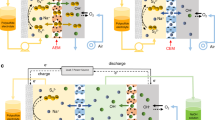
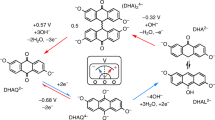
Aqueous redox flow battery (RFB) is one of the most promising technologies for grid-scale energy storage systems. Polysulfides are particularly attractive active materials owing to their low cost and high capacity, but the low energy efficiency and low operating current density limit their practical applications. Here we report an active and durable molecule catalyst, riboflavin sodium phosphate (FMN-Na), to transform sluggish polysulfide reduction reactions to fast redox reactions of FMN-Na via homogeneous catalysis. The FMN-Na catalyst substantially reduces the overpotential of a polysulfide–ferrocyanide RFB (S-Fe RFB) from more than 800 mV to 241 mV at 30 mA cm−2. A catalysed S-Fe flow cell was demonstrated for 2,000 cycles at 40 mA cm−2 with a low decay rate of 0.00004% per cycle (0.0017% per day). A catalysed polysulfide–iodide RFB operated for 1,300 cycles under 40 mA cm−2 without capacity decay. This work addresses the bottleneck of polysulfide-based RFBs for long-duration energy storage applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets analysed and generated during the current study are included in the paper and its Supplementary Information.
References
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Yao, Y., Lei, J., Shi, Y., Ai, F. & Lu, Y.-C. Assessment methods and performance metrics for redox flow batteries. Nat. Energy 6, 582–588 (2021).
Yang, Z. et al. Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011).
Park, M., Ryu, J., Wang, W. & Cho, J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2, 16080 (2016).
Lei, J., Jiang, L. & Lu, Y.-C. Emerging aqueous manganese-based batteries: fundamental understanding, challenges, and opportunities. Chem. Phys. Rev. 4, 021307 (2023).
Ai, F. et al. Heteropoly acid negolytes for high-power-density aqueous redox flow batteries at low temperatures. Nat. Energy 7, 417–426 (2022).
Sum, E., Rychcik, M. & Skyllas-Kazacos, M. Investigation of the V(V)/V(IV) system for use in the positive half-cell of a redox battery. J. Power Sources 16, 85–95 (1985).
Lei, J., Yao, Y., Wang, Z. & Lu, Y.-C. Towards high-areal-capacity aqueous zinc–manganese batteries: promoting MnO2 dissolution by redox mediators. Energy Environ. Sci. 14, 4418–4426 (2021).
Skyllas‐Kazacos, M., Rychcik, M., Robins, R. G., Fane, A. G. & Green, M. A. New all‐vanadium redox flow cell. J. Electrochem. Soc. 133, 1057–1058 (1986).
Zhang, S. et al. Recent progress in polysulfide redox-flow batteries. Batter. Supercaps 2, 627–637 (2019).
Li, Z. et al. Air-breathing aqueous sulfur flow battery for ultralow-cost long-duration electrical storage. Joule 1, 306–327 (2017).
Xia, Y. et al. A cost-effective alkaline polysulfide-air redox flow battery enabled by a dual-membrane cell architecture. Nat. Commun. 13, 2388 (2022).
Su, L., Badel, A. F., Cao, C., Hinricher, J. J. & Brushett, F. R. Toward an inexpensive aqueous polysulfide–polyiodide redox flow battery. Ind. Eng. Chem. Res. 56, 9783–9792 (2017).
Liu, J. et al. Sulfur-based aqueous batteries: electrochemistry and strategies. J. Am. Chem. Soc. 143, 15475–15489 (2021).
Li, Z., Weng, G., Zou, Q., Cong, G. & Lu, Y.-C. A high-energy and low-cost polysulfide/iodide redox flow battery. Nano Energy 30, 283–292 (2016).
Lei, J., Yao, Y., Huang, Y. & Lu, Y.-C. A highly reversible low-cost aqueous sulfur–manganese redox flow battery. ACS Energy Lett. 8, 429–435 (2023).
Li, Z. & Lu, Y.-C. Polysulfide-based redox flow batteries with long life and low levelized cost enabled by charge-reinforced ion-selective membranes. Nat. Energy 6, 517–528 (2021).
Zhao, P., Zhang, H., Zhou, H. & Yi, B. Nickel foam and carbon felt applications for sodium polysulfide/bromine redox flow battery electrodes. Electrochim. Acta 51, 1091–1098 (2005).
Gao, M. et al. Successive ionic layer adsorption and reaction–deposited copper sulfide electrocatalyst for high-power polysulfide-based aqueous flow batteries. Mater. Today Energy 18, 100540 (2020).
Gross, M. M. & Manthiram, A. Aqueous polysulfide–air battery with a mediator-ion solid electrolyte and a copper sulfide catalyst for polysulfide redox. ACS Appl. Energy Mater. 1, 7230–7236 (2018).
Ma, D. et al. Highly active nanostructured CoS2/CoS heterojunction electrocatalysts for aqueous polysulfide/iodide redox flow batteries. Nat. Commun. 10, 3367 (2019).
Wei, X. et al. An aqueous redox flow battery based on neutral alkali metal ferri/ferrocyanide and polysulfide electrolytes. J. Electrochem. Soc. 163, A5150 (2015).
Stephens, I. E. L., Ducati, C. & Fray, D. J. Correlating microstructure and activity for polysulfide reduction and oxidation at WS2 electrocatalysts. J. Electrochem. Soc. 160, A757–A768 (2013).
Qin, Y., Li, X., Liu, W. & Lei, X. High-performance aqueous polysulfide-iodide flow battery realized by an efficient bifunctional catalyst based on copper sulfide. Mater. Today Energy 21, 100746 (2021).
Chen, B. et al. Doping engineering of M-N-C electrocatalyst based membrane-electrode assembly for high-performance aqueous polysulfides redox flow batteries. Adv. Sci. 10, 2206949 (2023).
Haapanen, O. & Sharma, V. A modeling and simulation perspective on the mechanism and function of respiratory complex I. Biochim. Biophys. Acta Bioenerg. 1859, 510–523 (2018).
Vanoni, M. A. Iron-sulfur flavoenzymes: the added value of making the most ancient redox cofactors and the versatile flavins work together. Open Biol. 11, 210010 (2021).
de Vries, S., Dörner, K., Strampraad, M. J. F. & Friedrich, T. Electron tunneling rates in respiratory complex I are tuned for efficient energy conversion. Angew. Chem. Int. Ed. 54, 2844–2848 (2015).
Durchschein, K., Hall, M. & Faber, K. Unusual reactions mediated by FMN-dependent ene- and nitro-reductases. Green. Chem. 15, 1764–1772 (2013).
Tan, S. L. J., Kan, J. M. & Webster, R. D. Differences in proton-coupled electron-transfer reactions of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) between buffered and unbuffered aqueous solutions. J. Phys. Chem. B 117, 13755–13766 (2013).
Orita, A., Verde, M. G., Sakai, M. & Meng, Y. S. A biomimetic redox flow battery based on flavin mononucleotide. Nat. Commun. 7, 13230 (2016).
Páez, T. et al. The redox-mediated nickel–metal hydride flow battery. Adv. Energy Mater. 12, 2102866 (2022).
Zhou, M. et al. Nernstian-potential-driven redox-targeting reactions of battery materials. Chem. 3, 1036–1049 (2017).
Chen, Y. et al. A stable and high-capacity redox targeting-based electrolyte for aqueous flow batteries. Joule 3, 2255–2267 (2019).
Feng, R. et al. Proton-regulated alcohol oxidation for high-capacity ketone-based flow battery anolyte. Joule 7, 1609–1622 (2023).
Heelis, P. F. The photophysical and photochemical properties of flavins (isoalloxazines). Chem. Soc. Rev. 11, 15–39 (1982).
Lee, K. J., Elgrishi, N., Kandemir, B. & Dempsey, J. L. Electrochemical and spectroscopic methods for evaluating molecular electrocatalysts. Nat. Rev. Chem. 1, 0039 (2017).
Helm, M. L., Stewart, M. P., Bullock, R. M., DuBois, M. R. & DuBois, D. L. A synthetic nickel electrocatalyst with a turnover frequency above 100,000 s−1 for H2 production. Science 333, 863–866 (2011).
Schöfberger, W. et al. A bifunctional electrocatalyst for oxygen evolution and oxygen reduction reactions in water. Angew. Chem. Int. Ed. 55, 2350–2355 (2016).
Kamyshny, A., Goifman, A., Gun, J., Rizkov, D. & Lev, O. Equilibrium distribution of polysulfide ions in aqueous solutions at 25 °C: a new approach for the study of polysulfides’ equilibria. Environ. Sci. Technol. 38, 6633–6644 (2004).
Hu, M., Wang, A. P., Luo, J., Wei, Q. & Liu, T. L. Cycling performance and mechanistic insights of ferricyanide electrolytes in alkaline redox flow batteries. Adv. Energy Mater. 13, 2203762 (2023).
Goulet, M.-A. & Aziz, M. J. Flow battery molecular reactant stability determined by symmetric cell cycling methods. J. Electrochem. Soc. 165, A1466 (2018).
Smith, S. B. & Bruice, T. C. Mechanisms of isoalloxazine (flavine) hydrolysis. J. Am. Chem. Soc. 97, 2875–2881 (1975).
Surrey, A. R. & Nachod, F. C. Alkaline hydrolysis of riboflavin. J. Am. Chem. Soc. 73, 2336–2338 (1951).
Grajek, H. Resonance energy transfer between FMN molecules in the presence of dimers: a review. J. Mol. Liq. 209, 169–186 (2015).
Zhang, D.-W., Tian, J., Chen, L., Zhang, L. & Li, Z.-T. Dimerization of conjugated radical cations: an emerging non-covalent interaction for self-assembly. Chem. Asian J. 10, 56–68 (2015).
Goulet, M.-A. et al. Extending the lifetime of organic flow batteries via redox state management. J. Am. Chem. Soc. 141, 8014–8019 (2019).
Kosower, E. M. & Cotter, J. L. Stable free radicals. II. The reduction of 1-methyl-4-cyanopyridinium ion to methylviologen cation radical. J. Am. Chem. Soc. 86, 5524–5527 (1964).
Chen, Y., Gao, X., Johnson, L. R. & Bruce, P. G. Kinetics of lithium peroxide oxidation by redox mediators and consequences for the lithium–oxygen cell. Nat. Commun. 9, 767 (2018).
Yang, Y.-M., Yu, H.-Z., Sun, X.-H. & Dang, Z.-M. Density functional theory calculations on S—S bond dissociation energies of disulfides. J. Phys. Org. Chem. 29, 6–13 (2016).
Kruse, H., Goerigk, L. & Grimme, S. Why the Standard B3LYP/6-31G* model chemistry should not be used in DFT calculations of molecular thermochemistry: understanding and correcting the problem. J. Org. Chem. 77, 10824–10834 (2012).
Frisch, M. J. et al. Gaussian 09, Revision D. 01 (Gaussian, Inc., 2013).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Acknowledgements
The work described in this paper was supported by Research Grants Council of the Hong Kong Special Administrative Region under grant nos. N_CUHK435/18 (received by Y.-C.L.), RFS2223-4S03 (received by Y.-C.L.) and C1002-21GF (received by Y.-C.L. and J.F.). Y.-C.L. acknowledges the support from Xplorer Prize by New Cornerstone Science Foundation.
Author information
Authors and Affiliations
Contributions
J.L. and Y.-C.L. conceived the projects, analysed the data and wrote the manuscript. J.L. conducted the electrochemical measurements and material characterizations. Y.Y., Y.S. and K.L.L. assisted with the electrochemical measurements. Y.Z. and J.F. conducted the theoretical calculation.
Corresponding author
Ethics declarations
Competing interests
J.L. and Y.-C.L. are inventors of a patent application (US Patent application number 17/705,171) on the molecular catalysts described herein. The other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Xiaodong Lei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–38, Notes 1 and 2, Table 1 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, J., Zhang, Y., Yao, Y. et al. An active and durable molecular catalyst for aqueous polysulfide-based redox flow batteries. Nat Energy 8, 1355–1364 (2023). https://doi.org/10.1038/s41560-023-01370-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01370-0