Abstract
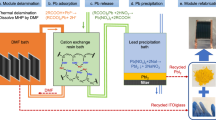
One major concern for the commercialization of perovskite photovoltaic technology is the toxicity of lead from the water-soluble lead halide perovskites that can contaminate the environment. Here, we report an abundant, low-cost and chemically robust cation-exchange resin (CER)-based method that can prevent lead leakage from damaged perovskite solar modules under severe weather conditions. CERs exhibit both high adsorption capacity and high adsorption rate of lead in water due to the high binding energy with lead ions in the mesoporous structure. Integrating CERs with carbon electrodes and layering them on the glass surface of modules has a negligible detrimental effect on device efficiency while reducing lead leakage from perovskite mini-modules by 62-fold to 14.3 ppb in water. The simulated lead leakage from damaged large-area perovskite solar panels treated with CERs can be further reduced to below 7.0 ppb even in the worst-case scenario that every sub-module is damaged.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published article and its Supplementary Information and Source Data files. Source data are provided with this paper.
References
NREL, Best Research-Cell Efficiency Chart https://www.nrel.gov/pv/cell-efficiency.html (accessed February 2020).
Deng, Y. et al. Tailoring solvent coordination for high-speed, room-temperature blading of perovskite photovoltaic films. Sci. Adv. 5, eaax7537 (2019).
Extance, A. The reality behind solar power’s next star material. Nature 570, 429–432 (2019).
Cheacharoen, R. et al. Encapsulating perovskite solar cells to withstand damp heat and thermal cycling. Sustain. Energy Fuels 2, 2398–2406 (2018).
Rong, Y. et al. Challenges for commercializing perovskite solar cells. Science 361, eaat8235 (2018).
Rajagopal, A., Yao, K. & Jen, A. K.-Y. Toward perovskite solar cell commercialization: a perspective and research roadmap based on interfacial engineering. Adv. Mater. 30, 1800455 (2018).
Ke, W. & Kanatzidis, M. G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 10, 965 (2019).
Kamat, P. V., Bisquert, J. & Buriak, J. Lead-free perovskite solar cells. ACS Energy Lett. 2, 904–905 (2017).
Yang, S. et al. Stabilizing halide perovskite surfaces for solar cell operation with wide-bandgap lead oxysalts. Science 365, 473–478 (2019).
Bai, S. et al. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 571, 245–250 (2019).
Jiang, Y. et al. Reduction of lead leakage from damaged lead halide perovskite solar modules using self-healing polymer-based encapsulation. Nat. Energy 4, 585–593 (2019).
Lee, J., Kim, G. W., Kim, M., Park, S. A. & Park, T. Nonaromatic green-solvent-processable, dopant-free, and lead-capturable hole transport polymers in perovskite solar cells with high efficiency. Adv. Energy Mater. 10, 1902662 (2020).
Li, X. et al. On-device lead sequestration for perovskite solar cells. Nature 578, 555–558 (2020).
Da̧browski, A., Hubicki, Z., Podkościelny, P. & Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56, 91–106 (2004).
Alexandratos, S. D. Ion-exchange resins: a retrospective from industrial and engineering chemistry research. Ind. Eng. Chem. Res. 48, 388–398 (2009).
Sulaeman, A., Driejana, D. & Hasan, N. Y. Composition of ions and trace metals in rainwater in Bandung city, Indonesia. IPTEK J. Proc. Ser. https://doi.org/10.12962/j23546026.y2017i6.3310 (2017).
Ropo, M., Schneider, M., Baldauf, C. & Blum, V. First-principles data set of 45,892 isolated and cation-coordinated conformers of 20 proteinogenic amino acids. Sci. Data 3, 160009 (2016).
Ropo, M., Blum, V. & Baldauf, C. Trends for isolated amino acids and dipeptides: conformation, divalent ion binding, and remarkable similarity of binding to calcium and lead. Sci. Rep. 6, 35772 (2016).
Florea, A.-M. et al. Lead (Pb2+) neurotoxicity: ion-mimicry with calcium (Ca2+) impairs synaptic transmission. A review with animated illustrations of the pre- and post-synaptic effects of lead. J. Local Glob. Health Sci. https://doi.org/10.5339/jlghs.2013.4 (2013).
Burgess, J. Metal Ions in Solution (Horwood, Chichester, 1978).
Razzaq, R., Shah, K. H., Fahad, M., Naeem, A. & Sherazi, T. A. Adsorption potential of macroporous Amberlyst-15 for Cd(II) removal from aqueous solutions. Mater. Res. Express 7, 025509 (2020).
Determining Resistance of Photovoltaic Modules to Hail by Impact With Propelled Ice Balls ASTM E1038-10, (ASTM International, 2019); https://www.astm.org/Standards/E1038.htm
Fagiolari, L. & Bella, F. Carbon-based materials for stable, cheaper and large-scale processable perovskite solar cells. Energy Environ. Sci. 12, 3437–3472 (2019).
Meng, F. N. et al. Current progress in interfacial engineering of carbon-based perovskite solar cells. J. Mater. Chem. A 7, 8690–8699 (2019).
Mei, A. et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science 345, 295–298 (2014).
Han, Y. et al. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A 3, 8139–8147 (2015).
Kato, Y. et al. Silver iodide formation in methyl ammonium lead iodide perovskite solar cells with silver top electrodes. Adv. Mater. Interfaces 2, 1500195 (2015).
Guerrero, A. et al. Interfacial degradation of planar lead halide perovskite solar cells. ACS Nano 10, 218–224 (2016).
Sanehira, E. M. et al. Influence of electrode interfaces on the stability of perovskite solar cells: reduced degradation using MoOx/Al for hole collection. ACS Energy Lett. 1, 38–45 (2016).
Grancini, G. et al. One-year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 8, 15684 (2017).
Chu, Q.-Q. et al. Highly stable carbon-based perovskite solar cell with a record efficiency of over 18% via hole transport engineering. J. Mater. Sci. Technol. 35, 987–993 (2019).
Wu, X. et al. Efficient and stable carbon-based perovskite solar cells enabled by the inorganic interface of CuSCN and carbon nanotubes. J. Mater. Chem. A 7, 12236–12243 (2019).
Arora, N. et al. Low-cost and highly efficient carbon-based perovskite solar cells exhibiting excellent long-term operational and UV stability. Small 15, 1904746 (2019).
Newbury, D.E. Mistakes encountered during automatic peak identification in low beam energy X-ray microanalysis. Scanning 29, 137–151 (2007).
Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungl. Svenska Vetenskapsakad. Handl. 24, 1–39 (1898).
Fairley, N. CasaXPS: processing software for XPS, AES, SIMS and more. v. 2, 15, (2016).
Blum, V. et al. Ab initio molecular simulations with numeric atom-centered orbitals. Comput. Phys. Commun. 180, 2175–2196 (2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Ambrosetti, A., Reilly, A. M., DiStasio, R. A. Jr. & Tkatchenko, A. Long-range correlation energy calculated from coupled atomic response functions. J. Chem. Phys. 140, 18A508 (2014).
Rossi, M., Chutia, S., Scheffler, M. & Blum, V. Validation challenge of density-functional theory for peptides—example of Ac-Phe-Ala5-LysH+. J. Phys. Chem. A 118, 7349–7359 (2014).
Schubert, F. et al. Exploring the conformational preferences of 20-residue peptides in isolation: Ac-Ala19-Lys + H+ vs. Ac-Lys-Ala19 + H+ and the current reach of DFT. Phys. Chem. Chem. Phys. 17, 7373–7385 (2015).
Acknowledgements
This research was financially supported mainly by the University of North Carolina Chapel Hill. We acknowledge support for the first-principles computations by the Center for Hybrid Organic Inorganic Semiconductors for Energy (CHOISE), an Energy Frontier Research Center (EFRC) funded by the US Department of Energy, Office of Basic Energy Sciences, Office of Science. We used the BET surface area and pore diameter analyser (Quantachrome NOVA 2000e) at the AMPED EFRC Instrumentation Facility established by the Alliance for Molecular PhotoElectrode Design for Solar Fuels, an EFRC funded by the US Department of Energy, Office of Basic Energy Sciences, Office of Science, under award number DE-SC0001011).
Author information
Authors and Affiliations
Contributions
J.H. and S.C. conceived the idea. S.C. fabricated the metal electrode PSCs and carbon perovskite solar devices, and conducted the lead leakage tests. Y.D. fabricated the metal electrode perovskite solar modules. H.G. prepared the carbon paste. S.X. simulated the lead leakage from solar panels. S.W. analysed the XPS results. Z.Y. assisted the fabrication of carbon PSCs. V.B. performed the computation of adsorption energies. J.H. and S.C. wrote the paper. All authors reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18 and Tables 1–3.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Rights and permissions
About this article
Cite this article
Chen, S., Deng, Y., Gu, H. et al. Trapping lead in perovskite solar modules with abundant and low-cost cation-exchange resins. Nat Energy 5, 1003–1011 (2020). https://doi.org/10.1038/s41560-020-00716-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-00716-2
This article is cited by
-
Mask-inspired moisture-transmitting and durable thermochromic perovskite smart windows
Nature Communications (2024)
-
Reducing lead toxicity of perovskite solar cells with a built-in supramolecular complex
Nature Sustainability (2023)
-
Hyperbranched polymer functionalized flexible perovskite solar cells with mechanical robustness and reduced lead leakage
Nature Communications (2023)
-
Room temperature nondestructive encapsulation via self-crosslinked fluorosilicone polymer enables damp heat-stable sustainable perovskite solar cells
Nature Communications (2023)
-
Preventing lead leakage in perovskite solar cells with a sustainable titanium dioxide sponge
Nature Sustainability (2023)