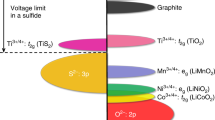
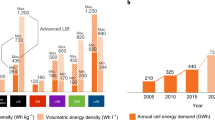
Abstract
Extreme fast charging, with a goal of 15 minutes recharge time, is poised to accelerate mass market adoption of electric vehicles, curb greenhouse gas emissions and, in turn, provide nations with greater energy security. However, the realization of such a goal requires research and development across multiple levels, with battery technology being a key technical barrier. The present-day high-energy lithium-ion batteries with graphite anodes and transition metal oxide cathodes in liquid electrolytes are unable to achieve the fast-charging goal without negatively affecting electrochemical performance and safety. Here we discuss the challenges and future research directions towards fast charging at the level of battery materials from mass transport, charge transfer and thermal management perspectives. Moreover, we highlight advanced characterization techniques to fundamentally understand the failure mechanisms of batteries during fast charging, which in turn would inform more rational battery designs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Karner, D., Garetson, T. & Francfort, J. EV Charging Infrastructure Roadmap (Idaho National Laboratory, 2016); https://inldigitallibrary.inl.gov/sites/sti/sti/7245706.pdf
Standard J1772: Electric Vehicle and Plug in Hybrid Electric Vehicle Conductive Charge Coupler (Society of Automotive Engineers, 2017).
Howell, D. et al. Enabling Fast Charging: A Technology Gap Assessment No. INL/EXT-17-41638 (US Department of Energy, 2017).
Ahmed, S. et al. Enabling fast charging — a battery technology gap assessment. J. Power Sources 367, 250–262 (2017). This work reviewed the developmental needs towards extreme fast charging at the battery cell and pack levels.
Keyser, M. et al. Enabling fast charging — battery thermal considerations. J. Power Sources 367, 228–236 (2017). This work reviewed thermal challenges with regards to extreme fast charging.
Meintz, A. et al. Enabling fast charging — vehicle considerations. J. Power Sources 367, 216–227 (2017).
Burnham, A. et al. Enabling fast charging — infrastructure and economic considerations. J. Power Sources 367, 237–249 (2017).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2009).
Nyman, A., Zavalis, T. G., Elger, R., Behm, M. & Lindbergh, G. Analysis of the polarization in a Li-ion battery cell by numerical simulations. J. Electrochem. Soc. 157, A1236–A1246 (2010).
Doyle, M., Fuller, T. F. & Newman, J. The importance of the lithium ion transference number in lithium/polymer cells. Electrochim. Acta 39, 2073–2081 (1994).
Gallagher, K. G. et al. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 163, A138–A149 (2016).
Logan, E. et al. A study of the physical properties of Li-ion battery electrolytes containing esters. J. Electrochem. Soc. 165, A21–A30 (2018).
Smart, M., Ratnakumar, B., Chin, K. & Whitcanack, L. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance. J. Electrochem. Soc. 157, A1361–A1374 (2010).
Smart, M., Ratnakumar, B. & Surampudi, S. Use of organic esters as cosolvents in electrolytes for lithium-ion batteries with improved low temperature performance. J. Electrochem. Soc. 149, A361–A370 (2002).
Lagadec, M. F., Zahn, R. & Wood, V. Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 4, 16–25 (2019).
Lee, H., Yanilmaz, M., Toprakci, O., Fu, K. & Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 7, 3857–3886 (2014).
Diederichsen, K. M., McShane, E. J. & McCloskey, B. D. Promising routes to a high Li+ transference number electrolyte for lithium ion batteries. ACS Energy Lett. 2, 2563–2575 (2017).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Videa, M., Xu, W., Geil, B., Marzke, R. & Angell, C. A. High Li+ self-diffusivity and transport number in novel electrolyte solutions. J. Electrochem. Soc. 148, A1352–A1356 (2001).
Popovic, J. et al. High lithium transference number electrolytes containing tetratriflylpropene’s lithium salt. J. Phys. Chem. Lett. 9, 5116–5120 (2018).
Buss, H. G., Chan, S. Y., Lynd, N. A. & McCloskey, B. D. Nonaqueous polyelectrolyte solutions as liquid electrolytes with high lithium ion transference number and conductivity. ACS Energy Lett. 2, 481–487 (2017).
Schaefer, J. L., Yanga, D. A. & Archer, L. A. High lithium transference number electrolytes via creation of 3-dimensional, charged, nanoporous networks from dense functionalized nanoparticle composites. Chem. Mater. 25, 834–839 (2013).
Suo, L., Hu, Y.-S., Li, H., Armand, M. & Chen, L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 4, 1481 (2013).
Varzi, A., Raccichini, R., Passerini, S. & Scrosati, B. Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries. J. Mater. Chem. A 4, 17251–17259 (2016).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Kerman, K., Luntz, A., Viswanathan, V., Chiang, Y.-M. & Chen, Z. Practical challenges hindering the development of solid state Li ion batteries. J. Electrochem. Soc. 164, A1731–A1744 (2017).
Hitz, G. T. et al. High-rate lithium cycling in a scalable trilayer Li-garnet-electrolyte architecture. Mater. Today 22, 50–57 (2019).
Zhang, H. et al. Single lithium-ion conducting solid polymer electrolytes: advances and perspectives. Chem. Soc. Rev. 46, 797–815 (2017).
Aetukuri, N. B. et al. Flexible ion-conducting composite membranes for lithium batteries. Adv. Energy Mater. 5, 1500265 (2015).
Porcarelli, L. et al. Single-ion conducting polymer electrolytes for lithium metal polymer batteries that operate at ambient temperature. ACS Energy Lett. 1, 678–682 (2016).
Oh, H. et al. Poly(arylene ether)-based single-ion conductors for lithium-ion batteries. Chem. Mater. 28, 188–196 (2015).
Harris, S. J. & Lu, P. Effects of inhomogeneities — nanoscale to mesoscale — on the durability of Li-ion batteries. J. Phys. Chem. C. 117, 6481–6492 (2013).
Thorat, I. V. et al. Quantifying tortuosity in porous Li-ion battery materials. J. Power Sources 188, 592–600 (2009).
Billaud, J., Bouville, F., Magrini, T., Villevieille, C. & Studart, A. R. Magnetically aligned graphite electrodes for high-rate performance Li-ion batteries. Nat. Energy 1, 16097 (2016).
Bae, C. J., Erdonmez, C. K., Halloran, J. W. & Chiang, Y. M. Design of battery electrodes with dual-scale porosity to minimize tortuosity and maximize performance. Adv. Mater. 25, 1254–1258 (2013).
Behr, S., Amin, R., Chiang, Y. & Tomsia, A. Highly-structured, additive-free lithium-ion cathodes by freeze-casting technology. Ceram. Forum Int. 92, 39–43 (2015).
Sander, J., Erb, R. M., Li, L., Gurijala, A. & Chiang, Y.-M. High-performance battery electrodes via magnetic templating. Nat. Energy 1, 16099 (2016).
Long, J. W., Dunn, B., Rolison, D. R. & White, H. S. Three-dimensional battery architectures. Chem. Rev. 104, 4463–4492 (2004).
Pikul, J. H., Zhang, H. G., Cho, J., Braun, P. V. & King, W. P. High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 4, 1732 (2013).
Li, G. et al. Stable metal battery anodes enabled by polyethylenimine sponge hosts by way of electrokinetic effects. Nat. Energy 3, 1076–1083 (2018).
Chu, H.-C. & Tuan, H.-Y. High-performance lithium-ion batteries with 1.5 μm thin copper nanowire foil as a current collector. J. Power Sources 346, 40–48 (2017).
Jow, T. R., Delp, S. A., Allen, J. L., Jones, J.-P. & Smart, M. C. Factors limiting Li+ charge transfer kinetics in Li-ion batteries. J. Electrochem. Soc. 165, A361–A367 (2018).
Abe, T., Sagane, F., Ohtsuka, M., Iriyama, Y. & Ogumi, Z. Lithium-ion transfer at the interface between lithium-ion conductive ceramic electrolyte and liquid electrolyte — a key to enhancing the rate capability of lithium-ion batteries. J. Electrochem. Soc. 152, A2151–A2154 (2005).
Xu, K., von Cresce, A. & Lee, U. Differentiating contributions to “ion transfer” barrier from interphasial resistance and Li+ desolvation at electrolyte/graphite interface. Langmuir 26, 11538–11543 (2010).
Peled, E. & Menkin, S. SEI: past, present and future. J. Electrochem. Soc. 164, A1703–A1719 (2017).
Liu, Y. et al. Solubility-mediated sustained release enabling nitrate additive in carbonate electrolytes for stable lithium metal anode. Nat. Commun. 9, 3656 (2018).
Komaba, S., Ozeki, T. & Okushi, K. Functional interface of polymer modified graphite anode. J. Power Sources 189, 197–203 (2009).
Ming, J. et al. New insights on graphite anode stability in rechargeable batteries: Li ion coordination structures prevail over solid electrolyte interphases. ACS Energy Lett. 3, 335–340 (2018).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017). This work demonstrated the use of cryo-electron microscopy for characterizing battery materials.
Wang, X. et al. New insights on the structure of electrochemically deposited lithium metal and its solid electrolyte interphases via cryogenic TEM. Nano Lett. 17, 7606–7612 (2017).
Zachman, M. J., Tu, Z., Choudhury, S., Archer, L. A. & Kourkoutis, L. F. Cryo-STEM mapping of solid-liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345–349 (2018).
Li, F. S., Wu, Y. S., Chou, J., Winter, M. & Wu, N. L. A mechanically robust and highly ion-conductive polymer-blend coating for high-power and long-life lithium-ion battery anodes. Adv. Mater. 27, 130–137 (2015).
Wang, C., Zhao, H., Wang, J., Wang, J. & Lv, P. Electrochemical performance of modified artificial graphite as anode material for lithium ion batteries. Ionics 19, 221–226 (2013).
Persson, K. et al. Lithium diffusion in graphitic carbon. J. Phys. Chem. Lett. 1, 1176–1180 (2010).
Cheng, Q., Yuge, R., Nakahara, K., Tamura, N. & Miyamoto, S. KOH etched graphite for fast chargeable lithium-ion batteries. J. Power Sources 284, 258–263 (2015).
Kim, N., Chae, S., Ma, J., Ko, M. & Cho, J. Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes. Nat. Commun. 8, 812 (2017). This work demonstrated a fast-charging hybrid anode of an amorphous silicon nanolayer and edge-site-activated graphite.
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Yan, K. et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 16010 (2016).
Sun, Y. et al. Graphite-encapsulated Li-metal hybrid anodes for high-capacity Li batteries. Chem 1, 287–297 (2016).
Burns, J., Stevens, D. & Dahn, J. In-situ detection of lithium plating using high precision coulometry. J. Electrochem. Soc. 162, A959–A964 (2015).
Downie, L. et al. In situ detection of lithium plating on graphite electrodes by electrochemical calorimetry. J. Electrochem. Soc. 160, A588–A594 (2013).
Wu, H., Zhuo, D., Kong, D. & Cui, Y. Improving battery safety by early detection of internal shorting with a bifunctional separator. Nat. Commun. 5, 5193 (2014).
Liu, K. et al. Electrospun core–shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 3, e1601978 (2017).
Takami, N., Satoh, A., Hara, M. & Ohsaki, T. Structural and kinetic characterization of lithium intercalation into carbon anodes for secondary lithium batteries. J. Electrochem. Soc. 142, 371–379 (1995).
Severson, K. A. et al. Data-driven prediction of battery cycle life before capacity degradation. Nat. Energy 4, 383–391 (2019). This work utlized machine learning to predict battery lives based on data collected from the early stages of battery cycling.
Nitta, N., Wu, F., Lee, J. T. & Yushin, G. Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015).
Jung, H.-G., Jang, M. W., Hassoun, J., Sun, Y.-K. & Scrosati, B. A high-rate long-life Li4Ti5O12/Li[Ni 0.45Co0.1Mn1.45]O4 lithium-ion battery. Nat. Commun. 2, 516 (2011).
Han, J.-T., Huang, Y.-H. & Goodenough, J. B. New anode framework for rechargeable lithium batteries. Chem. Mater. 23, 2027–2029 (2011).
Takami, N. et al. High-energy, fast-charging, long-life lithium-ion batteries using TiNb2O7 anodes for automotive applications. J. Power Sources 396, 429–436 (2018).
Son, I. H. et al. Silicon carbide-free graphene growth on silicon for lithium-ion battery with high volumetric energy density. Nat. Commun. 6, 7393 (2015).
Sun, J. et al. A phosphorene–graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 10, 980–985 (2015).
Li, W. et al. Amorphous red phosphorus embedded in highly ordered mesoporous carbon with superior lithium and sodium storage capacity. Nano Lett. 16, 1546–1553 (2016).
Tang, Y., Zhang, Y., Li, W., Ma, B. & Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 44, 5926–5940 (2015).
Lin, D. et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 11, 626–632 (2016).
Zheng, J. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017).
Mao, C., Ruther, R. E., Li, J., Du, Z. & Belharouak, I. Identifying the limiting electrode in lithium ion batteries for extreme fast charging. Electrochem. Commun. 97, 37–41 (2018).
Li, Z., Huang, J., Liaw, B. Y., Metzler, V. & Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 254, 168–182 (2014).
Yang, X.-G., Zhang, G., Ge, S. & Wang, C.-Y. Fast charging of lithium-ion batteries at all temperatures. Proc. Natl Acad. Sci. USA 115, 7266–7271 (2018). This work demonstrated 15-minute fast charging of Li-ion batteries in cold-temperature environments by preheating the battery with internal heaters.
Nissan Leaf Owner’ s Manual (Nissan Motor, 2017); https://cdn.dealereprocess.net/cdn/servicemanuals/nissan/2017-leaf.pdf
Wang, C.-Y. et al. Lithium-ion battery structure that self-heats at low temperatures. Nature 529, 515–518 (2016).
Rodrigues, M.-T. F. et al. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2, 17108 (2017).
Leng, F., Tan, C. M. & Pecht, M. Effect of temperature on the aging rate of Li ion battery operating above room temperature. Sci. Rep. 5, 12967 (2015).
Ye, Y., Saw, L. H., Shi, Y., Somasundaram, K. & Tay, A. A. O. Effect of thermal contact resistances on fast charging of large format lithium ion batteries. Electrochim. Acta 134, 327–337 (2014).
Bandhauer, T. M., Garimella, S. & Fuller, T. F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 158, R1–R25 (2011).
Liu, K., Liu, Y., Lin, D., Pei, A. & Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 4, eaas9820 (2018).
W. Golubkov, A. et al. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 4, 3633–3642 (2014).
Yang, Y., Huang, X., Cao, Z. & Chen, G. Thermally conductive separator with hierarchical nano/microstructures for improving thermal management of batteries. Nano Energy 22, 301–309 (2016).
Hunt, I. A., Zhao, Y., Patel, Y. & Offer, G. J. Surface cooling causes accelerated degradation compared to tab cooling for lithium-ion pouch cells. J. Electrochem. Soc. 163, A1846–A1852 (2016).
Srinivasan, V. & Wang, C. Y. Analysis of electrochemical and thermal behavior of Li-ion cells. J. Electrochem. Soc. 150, A98–A106 (2003).
Tennessen, P. T., Weintraub, J. C. & Hermann, W. A. Extruded and ribbed thermal interface for use with a battery cooling system. US patent US8758924B2 (2014).
Hao, M., Li, J., Park, S., Moura, S. & Dames, C. Efficient thermal management of Li-ion batteries with a passive interfacial thermal regulator based on a shape memory alloy. Nat. Energy 3, 899–906 (2018).
Lu, J., Wu, T. & Amine, K. State-of-the–art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2, 17011 (2017).
Hu, E., Wang, X., Yu, X. & Yang, X.-Q. Probing the complexities of structural changes in layered oxide cathode materials for Li-ion batteries during fast charge-discharge cycling and heating. Acc. Chem. Res. 51, 290–298 (2018). This work reviewed major characterization techniques at multilength scales for monitoring the structural evolution and kinetic characteristics of layered transition metal oxides during fast charge/discharge.
Xia, S. et al. Chemomechanical interplay of layered cathode materials undergoing fast charging in lithium batteries. Nano Energy 53, 753–762 (2018).
Wood, V. X-ray tomography for battery research and development. Nat. Rev. Mater. 3, 293–295 (2018).
Liu, H. et al. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes. Science 344, 1252817 (2014).
Zhou, Y. N. et al. High-rate charging induced intermediate phases and structural changes of layer-structured cathode for lithium-ion batteries. Adv. Energy Mater. 6, 1600597 (2016).
Lim, J. et al. Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 353, 566–571 (2016).
Oddershede, J. et al. Determining grain resolved stresses in polycrystalline materials using three-dimensional X-ray diffraction. J. Appl. Crystallogr. 43, 539–549 (2010).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy under the eXtreme Fast Charge Cell Evaluation of Li-ion batteries (XCEL) programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Zhu, Y. & Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat Energy 4, 540–550 (2019). https://doi.org/10.1038/s41560-019-0405-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0405-3
This article is cited by
-
Sustainable plug-in electric vehicle integration into power systems
Nature Reviews Electrical Engineering (2024)
-
Origin of fast charging in hard carbon anodes
Nature Energy (2024)
-
Quadruple the rate capability of high-energy batteries through a porous current collector design
Nature Energy (2024)
-
Advances in 3D silicon-based lithium-ion microbatteries
Communications Materials (2024)
-
Low thermal contact resistance boron nitride nanosheets composites enabled by interfacial arc-like phonon bridge
Nature Communications (2024)