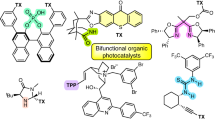
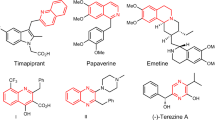
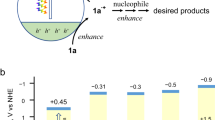
Abstract
During the past 15 years, an increasing number of research groups have embraced visible-light-mediated synthetic transformations as a powerful strategy for the construction and functionalization of organic molecules. This trend has followed the advent and development of photocatalysis, which often operates under mild visible-light irradiation. Nowadays, the general perception of UV-light photochemistry is often as an out-of-fashion approach that is difficult to perform and leads to unselective reaction pathways. Here we wish to propose an alternative and more realistic point of view to the scientific community. First, we will provide an overview of the use of UV light in modern photochemistry, highlighting the pivotal role it still plays in the development of new, efficient synthetic methods. We will then show how the high levels of mechanistic understanding reached for UV-light-driven processes have been key in the implementation of the related visible-light-driven transformations.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Roth, H. D. The beginnings of organic photochemistry. Angew. Chem. Int. Ed. 28, 1193–1207 (1989).
Ciamician, G. The photochemistry of the future. Science 36, 385–394 (1912).
Turro, N. J. & Schuster, G. Photochemical reactions as a tool in organic syntheses. Science 187, 303–312 (1975).
Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 108, 1052–1103 (2008).
Turro, N. J., Ramamurthy, V. & Scaiano, J. C. in Modern Molecular Photochemistry of Organic Molecules 319–382 (University Science Books, 2010).
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008).
Schultz, D. M. & Yoon, T. P. Solar synthesis: prospects in visible light photocatalysis. Science 343, 1239176 (2014).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Buglioni, L., Raymenants, F., Slattery, A., Zondag, S. D. A. & Noël, T. Technological innovations in photochemistry for organic synthesis: flow chemistry, high-throughput experimentation, scale-up and photoelectrochemistry. Chem. Rev. 122, 2752–2906 (2022).
Griesbeck, A. G., Abe, M. & Bondock, S. Selectivity control in electron spin inversion processes: regio- and stereochemistry of Paternò-Büchi photocycloadditions as a powerful tool for mapping intersystem crossing processes. Acc. Chem. Res. 37, 919–928 (2004).
Inoue, Y. Asymmetric photochemical reactions in solution. Chem. Rev. 92, 741–770 (1992).
Toda, F. Solid state organic chemistry: efficient reactions, remarkable yields and stereoselectivity. Acc. Chem. Res. 28, 480–486 (1995).
Ramamurthy, V. & Sivaguru, J. Supramolecular photochemistry as a potential synthetic tool: photocycloaddition. Chem. Rev. 116, 9914–9993 (2016).
Havinga, E. & Schlatmann, J. L. M. A. Remarks on the specificities of the photochemical and thermal transformations in the vitamin D field. Tetrahedron 16, 146–152 (1961).
Hammond, G. S. & Saltiel, J. Photosensitized cis-trans isomerization of the stilbenes. J. Am. Chem. Soc. 84, 4983–4984 (1962).
Ayitou, A. J.-L. & Sivaguru, J. Light-induced transfer of molecular chirality in solution: enantiospecific photocyclization of molecularly chiral acrylanilides. J. Am. Chem. Soc. 131, 5036–5037 (2009).
Kandappa, S. K. et al. Using restricted bond rotations to enforce excited-state behavior of organic molecules. Synlett 33, 1123–1134 (2022).
Ahuja, S., Raghunathan, R., Kumarasamy, E., Jockusch, S. & Sivaguru, J. Realizing the photoene reaction with alkenes under visible light irradiation and bypassing the favored [2 + 2]-photocycloaddition. J. Am. Chem. Soc. 140, 13185–13189 (2018).
Ayitou, A. J.-L. & Sivaguru, J. Reactive spin state dependent enantiospecific photocyclization of axially chiral α-substituted acrylanilides. Chem. Commun. 47, 2568–2570 (2011).
Inoue, Y. in Chiral Photochemistry Vol. 11 (eds Inoue, Y. & Ramamurthy, V.) 129–177 (CRC Press, 2004).
Oddy, M. J., Kusza, D. A. & Petersen, W. F. Visible-light mediated metal-free 6π-photocyclization of N-acrylamides: thioxanthone triplet energy transfer enables the synthesis of 3,4-dihydroquinolin-2-ones. Org. Lett. 23, 8963–8967 (2021).
Kumarasamy, E., Jesuraj, J. L., Omlid, J. N., Ugrinov, A. & Sivaguru, J. Light-induced enantiospecific 4π ring closure of axially chiral 2-pyridones: enthalpic and entropic effects promoted by H-bonding. J. Am. Chem. Soc. 133, 17106–17109 (2011).
Ayitou, A. J.-L., Jesuraj, J. L., Barooah, N., Ugrinov, A. & Sivaguru, J. Enantiospecific photochemical Norrish/Yang type II reaction of nonbiaryl atropchiral α-oxoamides in solution—axial to point chirality transfer. J. Am. Chem. Soc. 131, 11314–11315 (2009).
Masuda, Y., Ishida, N. & Murakami, M. Light-driven carboxylation of o-alkylphenyl ketones with CO2. J. Am. Chem. Soc. 137, 14063–14066 (2015).
Dell’Amico, L., Vega-Peñaloza, A., Cuadros, S. & Melchiorre, P. Enantioselective organocatalytic Diels-Alder trapping of photochemically generated hydroxy-o-quinodimethanes. Angew. Chem. Int. Ed. 55, 3313–3317 (2016).
Dell’Amico, L., Fernández-Alvarez, V. M., Maseras, F. & Melchiorre, P. Light-driven enantioselective organocatalytic β-benzylation of enals. Angew. Chem. Int. Ed. 56, 3304–3308 (2017).
Cuadros, S., Dell’Amico, L. & Melchiorre, P. Forging fluorine-containing quaternary stereocenters by a light-driven organocatalytic aldol desymmetrization process. Angew. Chem. Int. Ed. 56, 11875–11879 (2017).
Mateos, J. et al. A microfluidic photoreactor enables 2-methylbenzophenone light-driven reactions with superior performance. Chem. Commun. 54, 6820–6823 (2018).
Mateos, J. et al. Naphthochromenones: organic bimodal photocatalysts engaging in both oxidative and reductive quenching processes. Angew. Chem. Int. Ed. 59, 1302–1312 (2020).
Plutschack, M. B., Pieber, B., Gilmore, K. & Seeberger, P. H. The Hitchhiker’s Guide to flow chemistry parallel. Chem. Rev. 117, 11796–11893 (2017).
Woo, J. et al. Scaffold hopping by net photochemical carbon deletion of azaarenes. Science 376, 527–532 (2022).
Albini, A. & Alpegiani, M. The photochemistry of the N-oxide function. Chem. Rev. 84, 43–71 (1984).
Mateos, J. et al. Unveiling the impact of the light source and steric factors on [2 + 2] heterocycloaddition reactions. Nat. Synth. 2, 26–36 (2023).
Austin, K. A. B., Herdtweck, E. & Bach, T. Intramolecular [2 + 2] photocycloaddition of substituted isoquinolones: enantioselectivity and kinetic resolution induced by a chiral template. Angew. Chem. Int. Ed. 50, 8416–8419 (2011).
Coote, S. C. & Bach, T. Enantioselective intermolecular [2 + 2] photocycloadditions of isoquinolone mediated by a chiral hydrogen-bonding template. J. Am. Chem. Soc. 135, 14948–14951 (2013).
Brenninger, C., Jolliffe, J. D. & Bach, T. Chromophore activation of α,β-unsaturated carbonyl compounds and its application to enantioselective photochemical reactions. Angew. Chem. Int. Ed. 57, 14338–14349 (2018).
Brimioulle, R. & Bach, T. Enantioselective Lewis acid catalysis of intramolecular enone [2 + 2] photocycloaddition reactions. Science 342, 840–843 (2013).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Juris, A. et al. Ru(II) polypyridine complexes: photophysics, photochemistry, eleclrochemistry and chemiluminescence. Coord. Chem. Rev. 84, 85–277 (1988).
Lowry, M. S. et al. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem. Mater. 17, 5712–5719 (2005).
Timpe, H.-J. & Kronfeld, K.-P. Light-induced polymer and polymerization reactions XXXIII: direct photoinitiation of methyl methacrylate polymerization by excited states of ketones. J. Photochem. Photobiol. A 46, 253–267 (1989).
Kumarasamy, E. et al. Transposed Paternò–Büchi reaction. J. Am. Chem. Soc. 139, 655–662 (2017).
Kumarasamy, E., Kandappa, S. K., Raghunathan, R., Jockusch, S. & Sivaguru, J. Realizing an aza Paternò–Büchi reaction. Angew. Chem. Int. Ed. 56, 7056–7061 (2017).
Becker, M. R., Richardson, A. D. & Schindler, C. S. Functionalized azetidines via visible light-enabled aza Paternò–Büchi reactions. Nat. Commun. 10, 5095 (2019).
Kandappa, S. K., Valloli, L. K., Ahuja, S., Parthiban, J. & Sivaguru, J. Taming the excited state reactivity of imines—from non-radiative decay to aza Paternò–Büchi reaction. Chem. Soc. Rev. 50, 1617–1641 (2021).
Kumagai, T., Kawamura, Y. & Mukai, T. Photochemical reaction of 3-aryl-2-isoxazolines with methylated benzenes. Tetrahedron Lett. 24, 2279–2282 (1983).
Becker, M. R., Wearing, E. R. & Schindler, C. S. Synthesis of azetidines via visible-light-mediated intermolecular [2 + 2] photocycloadditions. Nat. Chem. 12, 898–905 (2020).
Poplata, S., Tröster, A., Zou, Y.-Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2 + 2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Liebermann, C. Ueber polythymochinon. Ber. Dtsch. Chem. Ges. 10, 2177–2179 (1877).
Gamlin, J. N. et al. The ionic auxiliary concept in solid state organic photochemistry. Acc. Chem. Res. 29, 203–209 (1996).
Brimioulle, R., Bauer, A. & Bach, T. Enantioselective Lewis acid catalysis in intramolecular [2 + 2] photocycloaddition reactions: a mechanistic comparison between representative coumarin and enone substrates. J. Am. Chem. Soc. 137, 5170–5176 (2015).
Vallavoju, N., Selvakumar, S., Jockusch, S., Sibi, M. P. & Sivaguru, J. Enantioselective organo-photocatalysis mediated by atropisomeric thiourea derivatives. Angew. Chem. Int. Ed. 53, 5604–5608 (2014).
Blum, T. R., Miller, Z. D., Bates, D. M., Guzei, I. A. & Yoon, T. P. Enantioselective photochemistry through Lewis acid-catalyzed triplet energy transfer. Science 354, 1391–1395 (2016).
Kumarasamy, E., Raghunathan, R., Jockusch, S., Ugrinov, A. & Sivaguru, J. Tailoring atropisomeric maleimides for stereospecific [2 + 2] photocycloaddition—photochemical and photophysical investigations leading to visible-light photocatalysis. J. Am. Chem. Soc. 136, 8729–8737 (2014).
Prinzbach, H., Eberbach, W. & von Veh, G. Photochemical isomerization of the tricyclo [3,2,1,02,4]octene system—a homovinylcyclopropane system. Angew. Chem. Int. Ed. 4, 436–437 (1965).
Kleinmans, R. et al. Intermolecular [2π + 2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Gozem, S., Luk, H. L., Schapiro, I. & Olivucci, M. Theory and simulation of the ultrafast double-bond isomerization of biological chromophores. Chem. Rev. 117, 13502–13565 (2017).
Turro, N. J., Ramamurthy, V. & Scaiano, J. C. in Modern Molecular Photochemistry of Organic Molecules 705–746 & 1018–1031 (University Science Books, 2010).
Nevesely, T., Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E → Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2022).
Molloy, J. J. et al. Boron-enabled geometric isomerization of alkenes via selective energy-transfer catalysis. Science 369, 302–306 (2020).
Adorinni, S. et al. Self-assembly of benzophenone-diphenylalanine conjugate into a nanostructured photocatalyst. Chem. Commun. 59, 7619–7622 (2023).
Drucker, C. S., Toscano, V. G. & Weiss, R. G. General method for the determination of steric effects during collisional energy transfer. Partial photoresolution of penta-2,3-diene. J. Am. Chem. Soc. 95, 6482–6484 (1973).
Hölzl-Hobmeier, A. et al. Catalytic deracemization of chiral allenes by sensitized excitation with visible light. Nature 564, 240–243 (2018).
De Mayo, P., Takeshita, H. & Sattar, A. B. M. A. The photochemical synthesis of 1,5-diketones and their cyclisation: a new annulation process. Proc. Chem. Soc. 119, D0376A (1962).
De Mayo, P. Enone photoannelation. Acc. Chem. Res. 4, 41–47 (1971).
Disanayaka, B. W. & Weedon, A. C. Application of the de Mayo reaction to the preparation of tricyclo[6.3.0.02,6]undecanes: a photochemical synthesis of (±)-hirsutene. J. Org. Chem. 52, 2905–2910 (1987).
Zhang, W., Zhang, L. & Luo, S. Catalytic asymmetric visible-light de Mayo reaction by ZrCl4-chiral phosphoric acid complex. J. Am. Chem. Soc. 145, 14227–14232 (2023).
Hasebe, M. & Tsuchiya, T. Photochemical generation of aliphatic radicals from benzophenone oxime esters: simple synthesis of alkylbenzenes and alkylpyridines. Tetrahedron Lett. 27, 3239–3242 (1986).
Soni, V. K. et al. Reactivity tuning for radical-radical cross-coupling via selective photocatalytic energy transfer: access to amine building blocks. ACS Catal. 9, 10454–10463 (2019).
Patra, T., Bellotti, P., Strieth-Kalthoff, F. & Glorius, F. Photosensitized intermolecular carboimination of alkenes through the persistent radical effect. Angew. Chem. Int. Ed. 59, 3172–3177 (2020).
Tan, G. et al. Photochemical single-step synthesis of beta-amino acid derivatives from alkenes and (hetero)arenes. Nat. Chem. 14, 1174–1184 (2022).
Protti, S. & Fagnoni, M. The sunny side of chemistry: green synthesis by solar light. Photochem. Photobiol. Sci. 8, 1499–1516 (2009).
Oelgemöller, M. Solar photochemical synthesis: from the beginnings of organic photochemistry to the solar manufacturing of commodity chemicals. Chem. Rev. 116, 9664–9682 (2016).
Williams, J. D. & Kappe, C. O. Recent advances toward sustainable flow photochemistry. Curr. Opin. Green Sustain. Chem. 25, 100351 (2020).
Schroeder, E. & Christopher, P. Chemical production using light: are sustainable photons cheap enough? ACS Energy Lett. 7, 880–884 (2022).
Acknowledgements
This work was supported by MUR (Ministero dell’Università) PRIN 2020927WY3_002 and European Research Council (ERC) Starting Grant 2021 SYNPHOCAT 101040025 (L.D.). G.G. thanks the MUR for a Young Researchers, Seal of Excellence fellowship (PNRR) funded by the European Union—NextGeneration EU. J.S. thanks the National Science Foundation for generous support for his research programme (CHE-1955524).
Author information
Authors and Affiliations
Contributions
L.D. and J.S. conceived the manuscript and identified the general concepts. The manuscript was written with contributions from all authors. All authors gave approval for the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Axel Griesbeck and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goti, G., Manal, K., Sivaguru, J. et al. The impact of UV light on synthetic photochemistry and photocatalysis. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01472-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01472-6