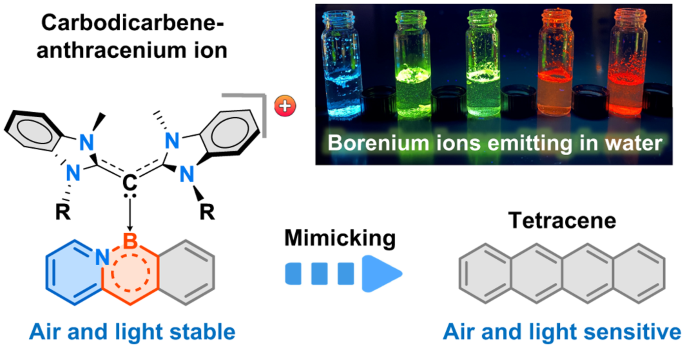
Abstract
Substitution of a C=C bond by an isoelectronic B–N bond is a well-established strategy to alter the electronic structure and stability of acenes. BN-substituted acenes that possess narrow energy gaps have attractive optoelectronic properties. However, they are susceptible to air and/or light. Here we present the design, synthesis and molecular structures of fully π-conjugated cationic BN-doped acenes stabilized by carbodicarbene ligands. They are luminescent in the solution and solid states and show high air and moisture stability. Compared with their neutral BN-substituted counterparts as well as the parent all-carbon acenes, these species display improved quantum yields and small optical gaps. The electronic structures of the azabora-anthracene and azabora-tetracene cations resemble higher-order acenes while possessing high photo-oxidative resistance. Investigations using density functional theory suggest that the stability and photo-physics of these conjugated systems may be ascribed to their cationic nature and the electronic properties of the carbodicarbene.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2225725 (1a), 2225726 (1b), 2225727 (2a), 2225728 (2b), 2225729 (3a), 2225730 (3b) and 2225731 (7). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other relevant data generated and analysed during this study, which include experimental, spectroscopic, crystallographic and computational data, are included in this article and its Supplementary Information. Source data are provided with this paper.
References
Anthony, J. E. Functionalized acenes and heteroacenes for organic electronics. Chem. Rev. 106, 5028–5048 (2006).
Chen, W. C., Lee, C. S. & Tong, Q. X. Blue-emitting organic electrofluorescence materials: progress and prospective. J. Mater. Chem. C 3, 10957–10963 (2015).
Zhu, M. R. & Yang, C. L. Blue fluorescent emitters: design tactics and applications in organic light-emitting diodes. Chem. Soc. Rev. 42, 4963–4976 (2013).
Ito, K. et al. Oligo(2,6-anthrylene)s: acene-oligomer approach for organic field-effect transistors. Angew. Chem. Int. Ed. 42, 1159–1162 (2003).
Tripathi, A. K., Heinrich, M., Siegrist, T. & Pflaum, J. Growth and electronic transport in 9,10-diphenylanthracene single crystals—an organic semiconductor of high electron and hole mobility. Adv. Mater. 19, 2097–2101 (2007).
Becker, H. D. Unimolecular photochemistry of anthracenes. Chem. Rev. 93, 145–172 (1993).
Anthony, J. E. The larger acenes: versatile organic semiconductors. Angew. Chem. Int. Ed. 47, 452–483 (2008).
Chien, C. T. et al. Tetracene-based field-effect transistors using solution processes. J. Mater. Chem. 22, 13070–13075 (2012).
Kitamura, M. & Arakawa, Y. Pentacene-based organic field-effect transistors. J. Phys. Condens. Matter 20, 184011 (2008).
Wang, Z. K., Naka, S. & Okada, H. Performance improvement of rubrene-based organic light emitting devices with a mixed single layer. Appl. Phys. A 100, 1103–1108 (2010).
Wu, T. C. et al. Singlet fission efficiency in tetracene-based organic solar cells. Appl. Phys. Lett. 104, 193901 (2014).
Wilson, M. W. B., Rao, A., Ehrler, B. & Friends, R. H. Singlet exciton fission in polycrystalline pentacene: from photophysics toward devices. Acc. Chem. Res. 46, 1330–1338 (2013).
Dong, S. Q., Ong, A. & Chi, C. Y. Photochemistry of various acene based molecules. J. Photochem. Photobiol. C 38, 27–46 (2019).
Zade, S. S. & Bendikov, M. Reactivity of acenes: mechanisms and dependence on acene length. J. Phys. Org. Chem. 25, 452–461 (2012).
Kouno, H. et al. Nonpentacene polarizing agents with improved air stability for triplet dynamic nuclear polarization at room temperature. J. Phys. Chem. Lett. 10, 2208–2213 (2019).
Kaur, I. et al. Substituent effects in pentacenes: gaining control over HOMO–LUMO gaps and photooxidative resistances. J. Am. Chem. Soc. 130, 16274–16286 (2008).
Abengozar, A., Garcia-Garcia, P., Fernandez-Rodriguez, M. A., Sucunza, D., & Vaquero, J. J. Recent developments in the chemistry of BN-aromatic hydrocarbons. Adv. Heterocycl. Chem. 135, 197–259 (2021).
Bosdet, M. J. D. & Piers, W. E. B–N as a C–C substitute in aromatic systems. Can. J. Chem. 87, 8–29 (2009).
Ishibashi, J. S. A., Darrigan, C., Chrostowska, A., Li, B. & Liu, S. Y. A BN anthracene mimics the electronic structure of more highly conjugated systems. Dalton Trans. 48, 2807–2812 (2019).
Ishibashi, J. S. A., Dargelos, A., Darrigan, C., Chrostowska, A. & Liu, S. Y. BN tetracene: extending the reach of BN/CC isosterism in acenes. Organometallics 36, 2494–2497 (2017).
Zhuang, F. D. et al. BN-embedded tetrabenzopentacene: a pentacene derivative with improved stability. Angew. Chem. Int. Ed. 58, 10708–10712 (2019).
Zhang, J. J. et al. Large acene derivatives with B–N Lewis pair doping: synthesis, characterization, and application. Org. Lett. 24, 1877–1882 (2022).
Dewar, M. J. S. & Tones, R. New heteroaromatic compounds part XXXI: the 12,11-borazarophenalenium cation. Tetrahedron Lett. 9, 2707–2708 (1968).
Gotoh, H. et al. Syntheses and physical properties of cationic BN-embedded polycyclic aromatic hydrocarbons. Angew. Chem. Int. Ed. 60, 12835–12840 (2021).
Ishikawa, Y., Suzuki, K. & Yamashita, M. 9-Aza-10-boraanthracene stabilized by coordination of an N-heterocyclic carbene and its methylated cation: synthesis, structure, and electronic properties. Organometallics 38, 2597–2601 (2019).
De Vries, T. S., Prokofjevs, A. & Vedejs, E. Cationic tricoordinate boron intermediates: borenium chemistry from the organic perspective. Chem. Rev. 112, 4246–4282 (2012).
Farrell, J. M., Hatnean, J. A. & Stephan, D. W. Activation of hydrogen and hydrogenation catalysis by a borenium cation. J. Am. Chem. Soc. 134, 15728–15731 (2012).
Farrell, J. M., Posaratnanathan, R. T. & Stephan, D. W. A family of N-heterocyclic carbene-stabilized borenium ions for metal-free imine hydrogenation catalysis. Chem. Sci. 6, 2010–2015 (2015).
Huang, Z. G. et al. Boron: its role in energy-related processes and applications. Angew. Chem. Int. Ed. 59, 8800–8816 (2020).
Nesterov, V. et al. NHCs in main group chemistry. Chem. Rev. 118, 9678–9842 (2018).
Baranac-Stojanovic, M. Aromaticity and stability of azaborines. Chem. Eur. J. 20, 16558–16565 (2014).
Klein, S., Tonner, R. & Frenking, G. Carbodicarbenes and related divalent carbon(0) compounds. Chem. Eur. J. 16, 10160–10170 (2010).
Dyker, C. A., Lavallo, V., Donnadieu, B. & Bertrand, G. Synthesis of an extremely bent acyclic allene (a ‘carbodicarbene’): A strong donor ligand. Angew. Chem. Int. Ed. 47, 3206–3209 (2008).
Liu, S., Chen, W C. & Ong, T. G. in Modern Ylide Chemistry. Structure and Bonding, 177 (ed Gessner, V.) (Springer, 2018).
Liu, S. K., Shih, W. C., Chen, W. C. & Ong, T. G. Carbodicarbenes and their captodative behavior in catalysis. ChemCatChem 10, 1483–1498 (2018).
Tonner, R. & Frenking, G. Divalent carbon(0) chemistry, part 2: protonation and complexes with main group and transition metal lewis acids. Chem. Eur. J. 14, 3273–3289 (2008).
Zhao, L. L., Chai, C. Q., Petz, W. & Frenking, G. Carbones and carbon atom as ligands in transition metal complexes. Molecules 25, 4943 (2020).
Aweke, B. S. et al. A bis-(carbone) pincer ligand and its coordinative behavior toward multi-metallic configurations. Angew .Chem. Int. Ed. 61, e202201884 (2022).
Chan, Y. C. et al. Synergistic catalysis by Brønsted acid/carbodicarbene mimicking frustrated Lewis pair-like reactivity. Angew. Chem. Int. Ed. 60, 19949–19956 (2021).
Chen, W. C. et al. The elusive three-coordinate dicationic hydrido boron complex. J. Am. Chem. Soc. 136, 914–917 (2014).
Chen, W. C. et al. Carbodicarbenes: unexpected π-accepting ability during reactivity with small molecules. J. Am. Chem. Soc. 139, 12830–12836 (2017).
Walley, J. E. et al. s-Block carbodicarbene chemistry: C(sp3)–H activation and cyclization mediated by a beryllium center. Chem. Commun. 55, 1967–1970 (2019).
Walley, J. E. et al. Carbodicarbene bismaalkene cations: unravelling the complexities of carbene versus carbone in heavy pnictogen chemistry. Angew. Chem. Int. Ed. 60, 6682–6690 (2021).
Hollister, K. K. et al. Air-stable thermoluminescent carbodicarbene-borafluorenium ions. J. Am. Chem. Soc. 144, 590–598 (2022).
Singh, S., Bhandari, M., Rawat, S. & Nembenna, S. in Polar Organometallic Reagents (eds Wheatley, A. E. H. & Uchiyama, M.) 201–269 (2022).
Franz, D. & Inoue, S. Cationic complexes of boron and aluminum: en early 21st century viewpoint. Chem. Eur. J. 25, 2898–2926 (2019).
Yang, Y. H., Gao, Q. & Xu, S. M. Ligand-free iridium-catalyzed dehydrogenative ortho C–H borylation of benzyl-2-pyridines at room temperature. Adv. Synth. Catal. 361, 858–862 (2019).
Chen, W. C., Hsu, Y. C., Lee, C. Y., Yap, G. P. A. & Ong, T. G. Synthetic modification of acyclic bent allenes (carbodicarbenes) and further studies on their structural implications and reactivities. Organometallics 32, 2435–2442 (2013).
Yang, W. L. et al. Stable borepinium and borafluorenium heterocycles: a reversible thermochromic ‘switch’ based on boron–oxygen interactions. Chem. Eur. J. 25, 12512–12516 (2019).
Allen, F. H. et al. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2, S1–S19 (1987).
Abbey, E. R., Zakharov, L. N. & Liu, S. Y. Crystal clear structural evidence for electron delocalization in 1,2-dihydro-1,2-azaborines. J. Am. Chem. Soc. 130, 7250–7252 (2008).
Costa, J. C. S., Taveira, R. J. S., Lima, C. F. R. A. C., Mendes, A. & Santos, L. M. N. B. F. Optical band gaps of organic semiconductor materials. Opt. Mater. 58, 51–60 (2016).
Sakamoto, Y. et al. Perfluoropentacene: high-performance p–n junctions and complementary circuits with pentacene. J. Am. Chem. Soc. 126, 8138–8140 (2004).
Northrop, B. H., Houk, K. N. & Maliakal, A. Photostability of pentacene and 6,13-disubstituted pentacene derivatives: a theoretical and experimental mechanistic study. Photochem. Photobiol. Sci, 7, 1463–1468 (2008).
Nijegorodov, N. & Winkoun, D. P. Dependence of the fluorescence parameters and the intersystem crossing rate constant on the orbital nature of the s(1) state of catacondensed aromatics. Spectrochim. Acta A 53, 2013–2022 (1997).
Burgdorff, C., Ehrhardt, S. & Lohmannsroben, H. G. Photophysical properties of tetracene derivatives in solution. 2. Halogenated tetracene derivatives. J. Phys. Chem. 95, 4246–4249 (1991).
Hestand, N. J. & Spano, F. C. Expanded theory of H- and J-molecular aggregates: the effects of vibronic coupling and intermolecular charge transfer. Chem. Rev. 118, 7069–7163 (2018).
Huang, Y. J. et al. Green grinding–coassembly engineering toward intrinsically luminescent tetracene in cocrystals. ACS Nano 14, 15962–15972 (2020).
Korovina, N. V., Pompetti, N. F. & Johnson, J. C. Lessons from intramolecular singlet fission with covalently bound chromophores. J. Chem. Phys. 152, 040904 (2020).
Paci, I. et al. Singlet fission for dye-sensitized solar cells: can a suitable sensitizer be found? J. Am. Chem. Soc. 128, 16546–16553 (2006).
Turro, N. J., Ramamurthy, V. & Scaiano, J. C. Modern Molecular Photochemistry of Organic Molecules (University Science Books, 2010).
Zhao, J. Z., Ji, S. M. & Guo, H. M. Triplet–triplet annihilation based upconversion: from triplet sensitizers and triplet acceptors to upconversion quantum yields. RSC Adv. 1, 937–950 (2011).
Ullrich, T., Munz, D. & Guldi, D. M. Unconventional singlet fission materials. Chem. Soc. Rev. 50, 3485–3518 (2021).
Ishibashi, J. S. et al. Two BN isosteres of anthracene: synthesis and characterization. J. Am. Chem. Soc. 136, 15414–15421 (2014).
Chan, S. C. et al. Observation of carbodicarbene ligand redox noninnocence in highly oxidized iron complexes. Angew. Chem. Int. Ed. 57, 15717–15722 (2018).
Liu, S.-k, Chen, W.-C., Yap, G. P. A. & Ong, T.-G. Synthesis of carbophosphinocarbene and their donating ability: expansion of the carbone class. Organometallics 39, 4395–4401 (2020).
Petz, W. & Frenking, G. in Transition Metal Complexes of Neutral η1-Carbon Ligands (eds Chauvin, R. & Canac, Y.) 49–92 (Springer, 2010).
Kuhn, N. & Kratz, T. Synthesis of imidazol-2-ylidenes by reduction of imidazole-2(3H)-thiones. Synthesis 1993, 561–562 (1993).
SAINT, APEX4 (Bruker AXS, 2019).
Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 48, 3–10 (2015).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Neese, F. The ORCA program system. Wires Comput. Mol. Sci. 2, 73–78 (2012).
Neese, F. Software update: the ORCA program system—version 5.0. Wires Comput. Mol. Sci. 12, e1606 (2022).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Schц╓fer, A., Huber, C. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Grimme, S. Semiempirical hybrid density functional with perturbative second-order correlation. J. Chem. Phys. 124, 034108 (2006).
Johnson, E. R. & Becke, A. D. A post-Hartree–Fock model of intermolecular interactions: Inclusion of higher-order corrections. J. Chem. Phys. 124, 174104 (2006).
Neese, F., Wennmohs, F., Hansen, A. & Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 356, 98–109 (2009).
Glendening, E. D., Landis, C. R. & Weinhold, F. NBO 6.0: natural bond orbital analysis program. J. Comput. Chem. 34, 1429–1437 (2013).
Knizia, G. Intrinsic atomic orbitals: an unbiased bridge between quantum theory and chemical concepts. J. Chem. Theory Comput. 9, 4834–4843 (2013).
Jensen, F. Segmented contracted basis sets optimized for nuclear magnetic shielding. J. Chem. Theory Comput. 11, 132–138 (2015).
Herges, R. & Geuenich, D. Delocalization of electrons in molecules. J. Phys. Chem. A 105, 3214–3220 (2001).
Schmider, H. L. & Becke, A. D. Chemical content of the kinetic energy density. J. Mol. Struct. Theochem. 527, 51–61 (2000).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, Inc., Wallingford CT, 2016).
Lu, T. & Chen, F. W. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Mitoraj, M. P., Michalak, A. & Ziegler, T. A combined charge and energy decomposition scheme for bond analysis. J. Chem. Theory Comput. 5, 962–975 (2009).
Radon, M. On the properties of natural orbitals for chemical valence. Theor. Chem. Acc. 120, 337–339 (2008).
Papajak, E., Zheng, J. J., Xu, X. F., Leverentz, H. R. & Truhlar, D. G. Perspectives on basis sets beautiful: seasonal plantings of diffuse basis functions. J. Chem. Theory Comput. 7, 3027–3034 (2011).
Zheng, J. J., Xu, X. F. & Truhlar, D. G. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 128, 295–305 (2011).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998).
Caricato, M. et al. Formation and relaxation of excited states in solution: a new time dependent polarizable continuum model based on time dependent density functional theory. J. Chem. Phys. 124, 124520 (2006).
Gao, X. et al. Evaluation of spin–orbit couplings with linear-response time-dependent density functional methods. J. Chem. Theory Comput. 13, 515–524 (2017).
Lee, T. J. & Taylor, P. R. A diagnostic for determining the quality of single-reference electron correlation methods. Int. J. Quantum Chem. 36, 199–207 (1989).
Riplinger, C., Sandhoefer, B., Hansen, A. & Neese, F. Natural triple excitations in local coupled cluster calculations with pair natural orbitals. J. Chem. Phys. 139, 134101 (2013).
Pople, J. A., HeadБ─Gordon, M. & Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 87, 5968–5975 (1987).
Kendall, R. A., Dunning, T. H. Jr. & Harrison, R. J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 96, 6796–6806 (1992).
Acknowledgements
We are grateful to the Arnold and Mabel Beckman Foundation for support of this work through a Beckman Young Investigator award (R.J.G.). The National Science Foundation Major Research Instrumentation (CHE 2018870) programme is also acknowledged (Bruker D8 Venture single crystal X-ray diffractometer). The University of Virginia (Rivanna) and Massachusetts Institute of Technology (Engaging, SuperCloud) High Performance Computing clusters provided computational resources and technical support that have contributed to the results reported herein.
Author information
Authors and Affiliations
Contributions
C.-L.D. and R.J.G. conceived and designed the project. C.-L.D. performed the experimental work as well as the theoretical studies. C.-L.D. and R.J.G. analysed the data. A.D.O. assisted in the synthetic experiments. B.Y.E.T. conducted the elemental analysis. S.K.S. provided the NHC ligand used in the experiments. D.A.D. and A.D.O. carried out the crystallographic data collection and refinement. C.-L.D. wrote the original draft, and R.J.G. edited with input from all authors. R.J.G. directed and supervised the research.
Corresponding author
Ethics declarations
Competing interests
C.-L.D., R.J.G., A.D.O. and S.K.S. are inventors on a provisional patent filed by the University of Virginia on the synthesis and properties of luminescent carbodicarbene-azaboraacenium ions.
Peer review
Peer review information
Nature Chemistry thanks Samuel Guieu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–116, Table 1–14, detailed synthetic procedures and NMR spectra for all compounds, crystallographic, photophysical, spectroscopic studies and computational details.
Supplementary Data 1
Crystallographic data for compound 1a, CCDC 2225725.
Supplementary Data 2
Crystallographic data for compound 1b, CCDC 2225726.
Supplementary Data 3
Crystallographic data for compound 2a, CCDC 2225727.
Supplementary Data 4
Crystallographic data for compound 2b, CCDC 2225728.
Supplementary Data 5
Crystallographic data for compound 3a, CCDC 2225729.
Supplementary Data 6
Crystallographic data for compound 3b, CCDC 2225730.
Supplementary Data 7
Crystallographic data for compound 7, CCDC 2225731.
Supplementary Data 8
Cartesian coordinates of optimized and calculated structures.
Supplementary Data 9
Source data for Supplementary Fig. 29, 30, 31, 32, 108b, 110b and 111b.
Source data
Source Data Fig. 5b
Numerical source data for Fig. 5b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, CL., Obi, A.D., Tra, B.Y.E. et al. Air- and photo-stable luminescent carbodicarbene-azaboraacenium ions. Nat. Chem. 16, 437–445 (2024). https://doi.org/10.1038/s41557-023-01381-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01381-0