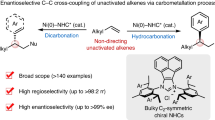
Abstract
Carbonyls and alkenes are versatile functional groups, whose reactivities are cornerstones of organic synthesis. The selective combination of two carbonyls to form an alkene—a carbonyl cross-metathesis—would be a valuable tool for their exchange. Yet, this important synthetic challenge remains unsolved. Although alkene/alkene and alkene/carbonyl cross-metathesis reactions are known, there is a lack of analogous methods for deoxygenative cross-coupling of two carbonyl compounds. Here we report a pair of strategies for the cross-metathesis of unbiased carbonyls, allowing an aldehyde to be chemo- and stereoselectively combined with another aldehyde or ketone. These mild, catalytic methods are promoted by earth-abundant metal salts and enable rapid access to an unprecedentedly broad range of either Z- or E-alkenes by two distinct mechanisms—entailing transiently generated (1) carbenes and ylides (via Fe catalysis) or (2) doubly nucleophilic gem-di-metallics (via Cr catalysis).

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are included in Supplementary Information.
References
Takeda, T. Modern carbonyl olefination—methods and applications. Synthesis 2004, 1532–1532 (2004).
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalysed olefin metathesis reaction. Nature 450, 243–251 (2007).
Albright, H. et al. Carbonyl-olefin metathesis. Chem. Rev. 121, 9359–9406 (2021).
Griffith, A. K., Vanos, C. M. & Lambert, T. H. Organocatalytic carbonyl-olefin metathesis. J. Am. Chem. Soc. 134, 18581–18584 (2012).
Ludwig, J. R., Zimmerman, P. M., Gianino, J. B. & Schindler, C. S. Iron(III)-catalysed carbonyl-olefin metathesis. Nature 533, 374–379 (2016).
Pitzer, L., Sandfort, F., Strieth‐Kalthoff, F. & Glorius, F. Carbonyl–olefin cross‐metathesis through a visible‐light‐induced 1,3‐diol formation and fragmentation sequence. Angew. Chem. Int. Ed. 57, 16219–16223 (2018).
McMurry, J. E. Carbonyl-coupling reactions using low-valent titanium. Chem. Rev. 89, 1513–1524 (1989).
McMurry, J. E. & Fleming, M. P. New method for the reductive coupling of carbonyls to olefins. Synthesis of β-carotene. J. Am. Chem. Soc. 96, 4708–4709 (1974).
Mukaiyama, T., Sato, T. & Hanna, J. Reductive coupling of carbonyl compounds to pinacols and olefins by using TiCl4 and Zn. Chem. Lett. 2, 1041–1044 (1973).
Asako, S. & Ilies, L. Olefin synthesis by deoxygenative coupling of carbonyl compounds: from stoichiometric to catalytic. Chem. Lett. 49, 1386–1393 (2020).
Bongso, A., Roswanda, R. & Syah, Y. M. Recent advances of carbonyl olefination via McMurry coupling reaction. RSC Adv. 12, 15885–15909 (2022).
McMurry, J. E., Fleming, M. P., Kees, K. L. & Krepski, L. R. Titanium-induced reductive coupling of carbonyls to olefins. J. Org. Chem. 43, 3255–3266 (1978).
Mukaiyama, T., Sugimura, H., Ohno, T. & Kobayashi, S. Reductive cross coupling reaction of a glyoxylate with carbonyl compounds. A facile synthesis of α,β-dihydroxycarboxylate based on a low valent titanium compound. Chem. Lett. 18, 1401–1404 (1989).
Wang, H., Dai, X. J. & Li, C. J. Aldehydes as alkyl carbanion equivalents for additions to carbonyl compounds. Nat. Chem. 9, 374–378 (2017).
Wei, W. et al. Ruthenium(II)-catalyzed olefination: via carbonyl reductive cross-coupling. Chem. Sci. 8, 8193–8197 (2017).
Esfandiarfard, K., Mai, J. & Ott, S. Unsymmetrical E-alkenes from the stereoselective reductive coupling of two aldehydes. J. Am. Chem. Soc. 139, 2940–2943 (2017).
Wang, S., Lokesh, N., Hioe, J., Gschwind, R. M. & König, B. Photoinitiated carbonyl-metathesis: deoxygenative reductive olefination of aromatic aldehydes via photoredox catalysis. Chem. Sci. 10, 4580–4587 (2019).
Wang, L., Lear, J. M., Rafferty, S. M., Fosu, S. C. & Nagib, D. A. Ketyl radical reactivity via atom transfer catalysis. Science 362, 225–229 (2018).
Yang, Z. P. & Fu, G. C. Convergent catalytic asymmetric synthesis of esters of chiral dialkyl carbinols. J. Am. Chem. Soc. 142, 5870–5875 (2020).
Rafferty, S. M., Rutherford, J. E., Zhang, L., Wang, L. & Nagib, D. A. Cross-selective aza-pinacol coupling via atom transfer catalysis. J. Am. Chem. Soc. 143, 5622–5628 (2021).
Huang, H.-M., Bellotti, P., Kim, S., Zhang, X. & Glorius, F. Catalytic multicomponent reaction involving a ketyl-type radical. Nat. Synth. 1, 464–474 (2022).
Huang, H. M., Bellotti, P., Erchinger, J. E., Paulisch, T. O. & Glorius, F. Radical carbonyl umpolung arylation via dual nickel catalysis. J. Am. Chem. Soc. 144, 1899–1909 (2022).
Zhu, C., Lee, S. C., Chen, H., Yue, H. & Rueping, M. Reductive cross-coupling of α-oxy halides enabled by thermal catalysis, photocatalysis, electrocatalysis, or mechanochemistry. Angew. Chem. Int. Ed. 61, e202204212 (2022).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Dong, Z. & MacMillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 598, 451–456 (2021).
Zhang, L., DeMuynck, B. M., Paneque, A. N., Rutherford, J. E. & Nagib, D. A. Carbene reactivity from alkyl and aryl aldehydes. Science 377, 649–654 (2022).
Aggarwal, V. K. & Winn, C. L. Catalytic, asymmetric sulfur ylide-mediated epoxidation of carbonyl compounds: scope, selectivity, and applications in synthesis. Acc. Chem. Res. 37, 611–620 (2004).
Ledford, B. E. & Carreira, E. M. Synthesis of unsaturated esters from aldehydes: an inexpensive, practical alternative to the Horner–Emmons reaction under neutral conditions. Tetrahedron Lett. 38, 8125–8128 (1997).
Lebel, H., Paquet, V. & Proulx, C. Methylenation of aldehydes: transition metal catalyzed formation of salt-free phosphorus ylides. Angew. Chem. Int. Ed. 40, 2887–2890 (2001).
Aggarwal, V. K., Fulton, J. R., Sheldon, C. G. & De Vicente, J. Generation of phosphoranes derived from phosphites. A new class of phosphorus ylides leading to high E selectivity with semi-stabilizing groups in Wittig olefinations. J. Am. Chem. Soc. 125, 6034–6035 (2003).
Filippini, D. & Silvi, M. Visible light-driven conjunctive olefination. Nat. Chem. 14, 66–70 (2022).
Takai, K., Hotta, Y., Oshima, K. & Nozaki, H. Effective methods of carbonyl methylenation using CH2I2–Zn–Me3Al and CH2Br2–Zn–TiCl4 system. Tetrahedron Lett. 19, 2417–2420 (1978).
Okazoe, T., Takai, K. & Utimoto, K. E-selective olefination of aldehydes by means of gem-dichromium reagents derived by reduction of gem-diiodoalkanes with chromium(II) chloride.J. Am. Chem. Soc. 109, 951–953 (1987).
Kochi, J. K. & Mocadlo, P. E. Facile reduction of alkyl halides with chromium(II) complexes. Alkylchromium species as intermediates. J. Am. Chem. Soc. 88, 4094–4096 (1966).
Marek, I. & Normant, J. F. Synthesis and reactivity of sp3-geminated organodimetallics. Chem. Rev. 96, 3241–3267 (1996).
Nallagonda, R., Padala, K. & Masarwa, A. gem-Diborylalkanes: recent advances in their preparation, transformation and application. Org. Biomol. Chem. 16, 1050–1064 (2018).
Banerjee, A. K., Sulbaran De Carrasco, M. C., Frydrych-Houge, C. S. V. & Motherwell, W. B. Observations on the reductive deoxygenation of aryl and α,β-unsaturated carbonyl compounds with chlorotrimethylsilane and zinc. J. Chem. Soc. Chem.Commun. 1986, 1803–1805 (1986).
Knecht, M. & Boland, W. E-selective alkylidenation of aldehydes with reagents derived from α-acetoxy bromides, zinc and CrCl3. Synlett 1993, 837–838 (1993).
Fürstner, A. & Shi, N. Nozaki–Hiyama–Kishi reactions catalytic in chromium. J. Am. Chem. Soc. 118, 12349–12357 (1996).
Zhao, K. & Knowles, R. R. Contra-thermodynamic positional isomerization of olefins. J. Am. Chem. Soc. 144, 137–144 (2022).
Zhang, C., Lin, Z., Zhu, Y. & Wang, C. Chromium-catalyzed allylic defluorinative ketyl olefin coupling. J. Am. Chem. Soc. 143, 11602–11610 (2021).
Schwarz, J. L., Schäfers, F., Tlahuext-Aca, A., Lückemeier, L. & Glorius, F. Diastereoselective allylation of aldehydes by dual photoredox and chromium catalysis. J. Am. Chem. Soc. 140, 12705–12709 (2018).
Ruffoni, A., Hampton, C., Simonetti, M. & Leonori, D. Photoexcited nitroarenes for the oxidative cleavage of alkenes. Nature 610, 81–86 (2022).
Wise, D. E. et al. Photoinduced oxygen transfer using nitroarenes for the anaerobic cleavage of alkenes. J. Am. Chem. Soc. 144, 15437–15442 (2022).
Acknowledgements
We thank the National Institutes of Health (R35 GM119812), National Science Foundation (CAREER 1654656) and Brown Science Foundation (BIA) for funding (to D.A.N.), and we are grateful to T. Bednar for independent verification of the robustness of this method.
Author information
Authors and Affiliations
Contributions
L.Z. and D.A.N. designed these strategies and wrote the manuscript. L.Z. performed all experiments and analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary procedures and characterization data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Nagib, D.A. Carbonyl cross-metathesis via deoxygenative gem-di-metal catalysis. Nat. Chem. 16, 107–113 (2024). https://doi.org/10.1038/s41557-023-01333-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01333-8