Abstract
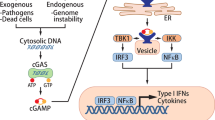
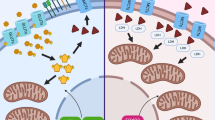
Evasion of antitumour immunity is a hallmark of cancer. STING, a putative innate immune signalling adaptor, has a pivotal role in mounting antitumour immunity by coordinating innate sensing and adaptive immune surveillance in myeloid cells. STING is markedly silenced in various human malignancies and acts as a cell-intrinsic tumour suppressor. How STING exerts intrinsic antitumour activity remains unclear. Here, we report that STING restricts aerobic glycolysis independent of its innate immune function. Mechanistically, STING targets hexokinase II (HK2) to block its hexokinase activity. As such, STING inhibits HK2 to restrict tumour aerobic glycolysis and promote antitumour immunity in vivo. In human colorectal carcinoma samples, lactate, which can be used as a surrogate for aerobic glycolysis, is negatively correlated with STING expression level and antitumour immunity. Taken together, this study reveals that STING functions as a cell-intrinsic metabolic checkpoint that restricts aerobic glycolysis to promote antitumour immunity. These findings have important implications for the development of STING-based therapeutic modalities to improve antitumour immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA-seq data supporting the findings of this study have been deposited in the Gene Expression Omnibus under accession code GSE202637. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Glickman, L. H. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cancer Res. 76, 1018–1030 (2016).
Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Zhang, Z.-D. & Zhong, B. Regulation and function of the cGAS-MITA/STING axis in health and disease. Cell Insight 1, 100001 (2022).
Yum, S., Li, M. H., Frankel, A. E. & Chen, Z. J. J. Roles of the cGAS-STING pathway in cancer immunosurveillance and immunotherapy. Annu. Rev. Cancer Biol. 3, 323–344 (2019).
Kwon, J. & Bakhoum, S. F. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 10, 26–39 (2020).
Sun, L. J., Wu, J. X., Du, F. H., Chen, X. & Chen, Z. J. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Wu, J. X. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013).
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Wang, H. et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl Acad. Sci USA 114, 1637–1642 (2017).
Liu, W. et al. Selective reactivation of STING signaling to target Merkel cell carcinoma. Proc. Natl Acad. Sci. USA 117, 13730–13739 (2020).
Song, S. S. et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci. Rep. 7, 39858 (2017).
Xia, T., Konno, H., Ahn, J. & Barber, G. N. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 14, 282–297 (2016).
Kitajima, S. et al. Suppression of STING associated with LKB1 loss in KRAS-driven lung cancer. Cancer Discov. 9, 34–45 (2019).
Xia, T., Konno, H. & Barber, G. N. Recurrent loss of STING signaling in melanoma correlates with susceptibility to viral oncolysis. Cancer Res. 76, 6747–6759 (2016).
Falahat, R. et al. STING signaling in melanoma cells shapes antigenicity and can promote antitumor T-cell activity. Cancer Immunol. Res. 7, 1837–1848 (2019).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004).
Lim, A. R., Rathmell, W. K. & Rathmell, J. C. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. eLife 9, e55185 (2020).
Reinfeld, B. I., Rathmell, W. K., Kim, T. K. & Rathmell, J. C. The therapeutic implications of immunosuppressive tumor aerobic glycolysis. Cell. Mol. Immunol. 19, 46–58 (2021).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK Cells. Cell Metab. 24, 657–671 (2016).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Gui, X. et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 567, 262–266 (2019).
Gonugunta, V. K. et al. Trafficking-mediated STING degradation requires sorting to acidified endolysosomes and can be targeted to enhance anti-tumor response. Cell Rep. 21, 3234–3242 (2017).
An, J., Woodward, J. J., Sasaki, T., Minie, M. & Elkon, K. B. Cutting edge: antimalarial drugs inhibit IFN-β production through blockade of cyclic GMP-AMP synthase-DNA interaction. J. Immunol. 194, 4089–4093 (2015).
Mukai, K. et al. Activation of STING requires palmitoylation at the Golgi. Nat. Commun. 7, 11932 (2016).
Kuchitsu, Y. et al. STING signalling is terminated through ESCRT-dependent microautophagy of vesicles originating from recycling endosomes. Nat. Cell Biol. 25, 453–466 (2023).
Sun, X. et al. DNA-PK deficiency potentiates cGAS-mediated antiviral innate immunity. Nat. Commun. 11, 6182 (2020).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015).
Schoggins, J. W. et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505, 691–695 (2014).
Bansal, A. & Simon, M. C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 217, 2291–2298 (2018).
Mattaini, K. R., Sullivan, M. R. & Vander Heiden, M. G. The importance of serine metabolism in cancer. J. Cell Biol. 214, 249–257 (2016).
Mukai, K. et al. Homeostatic regulation of STING by retrograde membrane traffic to the ER. Nat. Commun. 12, 61 (2021).
Deng, Z. et al. A defect in COPI-mediated transport of STING causes immune dysregulation in COPA syndrome. J. Exp. Med. 217, e20201045 (2020).
DeWaal, D. et al. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 9, 446 (2018).
Roberts, D. J. & Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 22, 248–257 (2015).
Ciscato, F. et al. Hexokinase 2 displacement from mitochondria-associated membranes prompts Ca2+-dependent death of cancer cells. EMBO Rep. 21, e49117 (2020).
Zhang, W. et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 178, 176–189 (2019).
Rath, A., Glibowicka, M., Nadeau, V. G., Chen, G. & Deber, C. M. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl Acad. Sci. USA 106, 1760–1765 (2009).
Shang, G., Zhang, C., Chen, Z. J., Bai, X. C. & Zhang, X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature 567, 389–393 (2019).
Patra, K. C. et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24, 213–228 (2013).
Pastorekova, S. & Gillies, R. J. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 38, 65–77 (2019).
Swietach, P., Patiar, S., Supuran, C. T., Harris, A. L. & Vaughan-Jones, R. D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J. Biol. Chem. 284, 20299–20310 (2009).
Patel, S. & Jin, L. TMEM173 variants and potential importance to human biology and disease. Genes Immun. 20, 82–89 (2019).
Hamann, L. et al. STING SNP R293Q is associated with a decreased risk of aging-related diseases. Gerontology 65, 145–154 (2019).
Falahat, R. et al. Epigenetic reprogramming of tumor cell-intrinsic STING function sculpts antigenicity and T cell recognition of melanoma. Proc. Natl Acad. Sci. USA 118, e2013598118 (2021).
Amouzegar, A., Chelvanambi, M., Filderman, J. N., Storkus, W. J. & Luke, J. J. STING agonists as cancer therapeutics. Cancers 13, 2695 (2021).
Le Naour, J., Zitvogel, L., Galluzzi, L., Vacchelli, E. & Kroemer, G. Trial watch: STING agonists in cancer therapy. Oncoimmunology 9, 1777624 (2020).
Akhmetova, K., Balasov, M. & Chesnokov, I. Drosophila STING protein has a role in lipid metabolism. eLife 10, e67358 (2021).
Jiang, H. et al. Chromatin-bound cGAS is an inhibitor of DNA repair and hence accelerates genome destabilization and cell death. EMBO J. 38, e102718 (2019).
Chen, H. et al. cGAS suppresses genomic instability as a decelerator of replication forks. Sci. Adv. 6, eabb8941 (2020).
Liu, H. P. et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 563, 131–136 (2018).
Contreras-Baeza, Y. et al. Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments. J. Biol. Chem. 294, 20135–20147 (2019).
Dobbs, N. et al. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe 18, 157–168 (2015).
Liu, Y. et al. Clathrin-associated AP-1 control stermination of STING signalling. Nature 610, 761–767 (2022).
Zhang, J. et al. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe 24, 234–248 (2018).
Luo, W. W. et al. iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nat. Immunol. 17, 1057–1066 (2016).
Zhang, J. J. et al. IκB kinase ε is an NFATc1 kinase that inhibits T cell immune response. Cell Rep. 16, 405–418 (2016).
Minassian, A. et al. An internally translated MAVS variant exposes its amino-terminal TRAF-binding motifs to deregulate interferon induction. PLoS Pathog. 11, e1005060 (2015).
Wang, Y. et al. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. Nat. Commun. 10, 201 (2019).
Wen, B., Mei, Z. L., Zeng, C. W. & Liu, S. Q. metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 18, 183 (2017).
Zhang, J. J. et al. Herpesviral G protein-coupled receptors activate NFAT to induce tumor formation via inhibiting the SERCA calcium ATPase. PLoS Pathog. 11, e1004768 (2015).
Acknowledgements
We thank J. Zhang, Q. Yang, B. Zhong, H. Liu, C. Cai, W. Song, H. Du, Z. J. Chen and X. Zhou for reagents; B. Zhong for suggestions; and the members of the core facility of the Medical Research Institute at Wuhan University for technical support. This work was supported by the National Key Research and Development Program of China 2021YFC2701800 (to J.Z.), 2021YFC2701804 (to J.Z.) and 2021YFC2701602 (to B.W.); a startup fund from Wuhan University (to J.Z.); National Natural Science Foundation of China 31970156 (to J.Z.), 82172261 (to J.Z.), 32188101 (to H.-B.S.) and 32170886 (to B.W.); the Fundamental Research Funds for the Central Universities (2042022dx0003) (to J.Z.) and 2042022rc0032 (to B.W.); and the Non-Profit Central Research Institute Fund of Chinese Academy of Medical Sciences 2020-PT320-004 (to J.Z.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.Z., P.F., B.W., H.-B.S. and J.Z. designed and supervised the study. L.Z., Y. Zhong, H.J., J.S. Y. Zhou and C.Z. performed most of the experiments. Y. Zhang and B.F. performed the IP–MS study. J.X. and M.T. analysed the sequencing and metabolomics data. K.S., X.S., S.W., X.C., W.W. and X.L. helped with animal experiments. C.J. and K.S. collected patient samples and helped with the related analysis. L.Z. and J.Z. wrote the paper. All of the authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Constantinos Koumenis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 STING restricts aerobic glycolysis.
a, Image of the colour of the cell culture media. b, the number of control or Sting1−/− L929 cells was quantified at the indicated time points. c, qPCR analysis of Isg56 or Cxcl10 mRNA in control or Sting1−/− L929 cells transfected with or without HT-DNA. d and e, immunoblotting analysis of L929 cells transfected with HT-DNA or infected with HSV-1. f, L929 cells were pre-treated with chloroquine (20 μM) or bafilomycin A1 (400 nM) for 30 minutes and then transfected with HT-DNA (1 μg/ml), followed by immunoblotting. g, L929 cells were infected with HSV-1 (MOI = 1) and treated with chloroquine (20 μM) or bafilomycin A1 (400 nM). WCLs were analysed by immunoblotting. h, L929 cells were pre-treated with chloroquine (20 μM) or bafilomycin A1 (400 nM) for 30 minutes and then transfected with HT-DNA (1 μg/ml). The expression of Cxcl10 and Isg56 was quantified at 8 h post-transfection. i, L929 cells were pre-treated with chloroquine (20 μM) or bafilomycin A1 (400 nM) for 30 minutes and then transfected with HT-DNA (1 μg/ml), followed by lactate meaturement. j, L929 cells were infected with HSV-1 (MOI = 1) and treated with chloroquine (20 μM) or bafilomycin A1 (400 nM). Lactate was measured at the indicated time points. k, L929 cells were pre-treated with brefeldin A (10 μg/ml) for 30 minutes and then transfected with HT-DNA (1 μg/ml). The expression of Cxcl10 and Isg56 was quantified at 8 h post-transfection. l, L929 cells were transfected with HT-DNA (1 μg/ml) and treated with brefeldin A (10 μg/ml). lactate was measured at 24 h post-transfection. Data are presented as means ± s.d. of n = 3 independent biological replicates for b, c, h-l. P-value was calculated by two-tailed Student’s t-test. Data are representative of three independent experiments. Uncropped gel images and numerical data are available in source data.
Extended Data Fig. 2 STING vesicles retained in the cytosol by Baf A1 or CQ restricts aerobic glycolysis.
a, L929 cells were transfected with control siRNA or Tsg101 siRNA, and the expression of Tsg101 was quantified at 72 h post-transfection. b, L929 cells from (a) were stimulated with HT-DNA (1 μg/ml) for 8 h. The expression of Cxcl10 and Isg56 was quantified by qPCR. c, L929 cells from (a) were stimulated with HT-DNA (1 μg/ml) for 24 h, followed by lactate measurement. d, L929 cells were transfected with Control siRNA or Atp6v1b2 siRNA, and the expression of Atp6v1b2 was quantified at 72 h post-transfection. e, Sting1−/− MLF cells stably expressing mRuby3-mSTING and Lamp1-EGFP were treated with control siRNA, Atp6v1b2 siRNA or Tsg101 siRNA for 60 h, and then stimulated with DMXAA (25 μg/ml) for 12 h. Cells were fixed and imaged by STED super-resolution microscopy. Scale bars, 5 μm. The boxed areas in the top panels were magnified in the bottom, and the fluorescence intensity profiles along the white dotted lines were shown. f, Sting1−/− MLF cells stably expressing mRuby3-mSTING and Lamp1-EGFP were stimulated with DMXAA (25 μg/ml), and treated with brefeldin A (10 μg/ml), chloroquine (20 μM), or bafilomycin A1 (400 nM) for 12 h. Cells were fixed and imaged by STED super-resolution microscopy. Scale bars, 5 μm. The boxed areas in the top panels were magnified in the bottom, and the fluorescence intensity profiles along the white dotted lines were shown. g, immunoblotting analysis of THP-1 cells infected with HSV-1 (MOI = 1). h and i, Sting1−/−L929 cells stably expressing FLAG-STING were transfected with HT-DNA (1 μg/ml) (h) or infected with HSV-1 (MOI = 1) (i), followed by immunoblotting. Data are presented as means ± s.d. of n = 3 independent biological replicates for a-d. P-value was calculated by two-tailed Student’s t-test. Data are representative of three independent experiments. Uncropped gel images and numerical data are available in source data.
Extended Data Fig. 3 STING suppresses lactate production independent of its innate immune function.
a, WT, Sting1−/− L929 cells and the reconstituted Sting1−/− L929 cells were analysed by immunoblotting. b, Sting1−/− L929 cells reconstituted with vector control, STING WT or the indicated mutants were stimulated with or without HT-DNA, and Isg56 or Cxcl10 mRNA was quantified at 6 h post-stimulation. c, immunoblotting analysis of STING-S366A reconstituted Sting1−/− L929 cells stimulated with HT-DNA or infected with HSV-1 for the indicated amount of time. d, immunoblotting analysis of STING-R238A reconstituted Sting1−/− L929 cells stimulated with HT-DNA or infected with HSV-1 for the indicated amount of time. e, Cgas−/− L929 cells reconstituted with vector control or cGAS were stimulated with or without HT-DNA, and Isg56 and Cxcl10 mRNA was quantified at 6 h post-stimulation. f and g, Immunoblotting of the reconstituted Cgas−/− L929 cells stimulated with HT-DNA (f) or infected with HSV-1 (g) for the indicated amount of time. h, L929 cells were transduced with control sgRNA or sgRNA targeting IRF3, and the stable cells were further transduced with control sgRNA or sg-STING to generate double knockout cells. WCLs were analysed by immunoblotting and lactate production was measured. i, Mouse lung fibroblasts (MLFs) isolated from WT or Ifnar1−/− mice were stimulated with or without Interferon-β (IFNβ), and Cxcl10 or Isg56 mRNA was quantified by qPCR at 6 h post-stimulation. j and k, WT and Ifnar1−/− MLF cells were transduced with control sgRNA or sg-STING. WCLs were analysed by immunoblotting (j) and lactate production was measured (k). Data are presented as means ± s.d. of n = 3 independent biological replicates for b, h, i, k, and n = 4 independent biological replicates for e. P-values were calculated by two-tailed Student’s t-test. Data are representative of three independent experiments. Uncropped gel images and numerical data are available in source data.
Extended Data Fig. 4 STING restricts aerobic glycolysis by targeting HK2.
a, RNA-seq was performed from control and Sting1−/− L929 cells. A heatmap shows the top 15 genes down-regulated in Sting1−/− L929 cells (n = 3 independent biological replicates). b, Heatmap of unsupervised hierarchical clustering of metabolites in control and Sting1−/− L929 cells. c, A volcano plot visualized metabolites that differ between control and Sting1−/− L929 cells, with glycolytic intermediates being annotated. d, [U-13C] -glucose stable isotope tracer analyses were performed in control and Sting1−/− L929 cells. The M3-labelled glycolysis intermediates (glycerol 3-phosphate, 3PG/2PG, Lactate) were displayed. Four replicates were analysed per experiment. e and f, L929 cells were transduced with control sgRNA or sgRNA targeting HK1 or HK2 to generate knockout cells. WCLs were analysed by immunoblotting (e), followed by the detection of lactate secretion and mitochondrial hexokinase activity (f). g, HEK293T cells were transfected with the indicated plasmids. WCLs were collected for immunoprecipitation with anti-FLAG affinity agarose, followed by immunoblotting analysis. h, Co-immunoprecipitation and immunoblotting of endogenous STING and HK2 in THP-1 cells. Anti-HK2 antibody was used for immunoprecipitation. Data are presented as means ± s.d. of n = 4 (d) or n = 3 (f) independent biological replicates. Statistical analyses were performed using a two-tailed unpaired Student’s t-test. Data are representative of three independent experiments. Source numerical data and unprocessed blots are provided in Source Data.
Extended Data Fig. 5 STING(1–160) associates with HK2 and suppresses HK2 activity.
a, L929 cells were transfected with control siRNA or Tsg101 siRNA for 60 h, and then stimulated with HT-DNA (1 μg/ml) for 12 h. Co-immunoprecipitation and immunoblot of endogenous STING and HK2 were shown as indicated. Anti-STING antibody was used for immunoprecipitation. b and c, L929 cells were pre-treated with brefeldin A (10 μg/ml), chloroquine (20 μM) or bafilomycin A1 (400 nM) for 30 minutes and then stimulated with HT-DNA (1 μg/ml) for 12 h. Co-immunoprecipitation and immunoblotting of endogenous STING and HK2 were shown as indicated. Anti-STING antibody was used for immunoprecipitation. d, the localization of HK2 and STING was visualized in MLF cells. Scale bars, 10 μm. The boxed areas in the top panels were magnified in the bottom, and the fluorescence intensity profile along the white dotted line was shown. e, Sting1−/− MLF cells stably expressing EGFP-mSTING were immunostained with anti-HK2 and anti-Calnexin (an ER marker) antibodies, and imaged by STED super-resolution microscopy. Scale bars, 5 μm. The boxed areas in the top panels were magnified in the bottom. f and g, Domain mapping of the interaction between STING and HK2. h, Analysis of hexokinase activity in purified mitochondria isolated from control and Cgas−/− L929 cells. i, Sting1−/− L929 cells were reconstituted with the indicated STING mutants, and mitochondrial hexokinase activity was analysed. j, Purified GST-HK2 was incubated with the indicated amount of purified STING, followed by the measurement of hexokinase activity. 2-DG (a hexokinase inhibitor) was a positive control. For h-j, Data are presented as means ± s.d. of n = 4 (h) or n = 3 (i and j) independent biological replicates. Statistical analyses were performed using a two-tailed unpaired Student’s t-test. Data are representative of three independent experiments. Source numerical data and unprocessed blots are provided in Source Data.
Extended Data Fig. 6 P2 of STING is required to associate with HK2 and suppress HK2 activity.
a, The Pearson’s correlation coefficients between HK2 and mitochondria. b, Sting1−/− MLF cells stably expressing EGFP-mSTING were labelled with MitoTracker and stimulated with DMXAA (25 μg/ml) for 30 min. After immunostaining with HK2 antibody, cells were imaged by STED super-resolution microscopy. Scale bars, 5 μm. c, Sting1−/− MLF cells stably expressing EGFP-mSTING were stimulated with DMXAA (25 μg/ml) for 30 min. After immunostaining with HK2 and GM130 (a Golgi marker) antibodies, cells were imaged by STED super-resolution microscopy. Scale bars, 5 μm. d and e, FLAG-HK2 and HA-tagged STING (1–160) or the indicated mutants were co-expressed in HEK293T cells. WCLs were immunoprecipitated with anti-FLAG agarose and analysed by immunoblotting. f, Reporter assays for IFNB1 promoter activity in HEK293T cells transfected with HA-tagged STING containing the indicated mutations and FLAG-cGAS (Left). WCLs were analysed by immunoblotting (Right). Data are presented as means ± s.d. of n = 8 (a) or n = 3 (f) independent biological replicates. Statistical analyses were performed using a two-tailed unpaired Student’s t-test. Data are representative of three independent experiments. Source numerical data and unprocessed blots are provided in Source Data.
Extended Data Fig. 7 STING stable expression in MC38 cells promotes antitumour immunity.
a, WCLs from L929, 293T, MC38, CT26 were analysed by immunoblotting. b, Representative MC38 tumour staining of Ki-67 and cleaved caspase-3 as described in Fig. 5b. The percentage of Ki-67- or cleaved caspase-3-positive cells was quantified (n = 6). Scale bars, 200 μm. c, Flow cytometry analysis of lymphocyte in MC38 tumours as described in Fig. 5b (n = 8). d, Flow cytometry analysis of tumour-infiltrating CD4+ T cells and the severely exhausted CD4+ cells in MC38 tumours as described in Fig. 5e (n = 4 Vector group, n = 5 STING group). e and f, Flow cytometry analysis of CD8+ and CD4+ T cells in the spleens of the MC38 tumour-bearing mice as described in Fig. 5e (n = 4 Vector group, n = 5 STING group). g, Representative MC38 tumour-bearing in NSG mice staining of Ki-67 and cleaved caspase-3 as described in Fig. 5f. The percentage of Ki-67- or cleaved caspase-3-positive cells was quantified (n = 6). Scale bars, 200 μm. Data are presented as means ± s.d., and statistical analyses were performed using a two-tailed unpaired Student’s t-test. Data are representative of three independent experiments. Source numerical data and unprocessed blots are provided in Source Data.
Extended Data Fig. 8 Depletion of STING in CT26 cells impairs antitumour immunity.
a, Representative CT26 tumour staining of Ki-67 and cleaved caspase-3 as described in Fig. 5k. The percentage of Ki-67- or cleaved caspase-3-positive cells was quantified (n = 6). Scale bars, 200 μm. b, Flow cytometry analysis of the tumour-infiltrating CD4+ T cells and the severe exhausted CD4+ T cells isolated from CT26 tumour and spleens as described in Fig. 5k (n = 6). c, Flow cytometry analysis of CD8+ and CD4+ T cells in the spleens of the CT26 tumour-bearing mice (n = 6). d-g, CT26 cells as described in Fig. 5i were subcutaneously transplanted into NSG mice (n = 8 per group). Tumour volume (d), weight (e), and lactate levels in the tumours at 10 days post-transplantation (f) were measured. IHC staining of Ki-67 and cleaved caspase-3 of the tumours were shown in (g). The percentage of Ki-67- or cleaved caspase-3-positive cells was quantified (n = 6). Scale bars, 200 μm. h, IHC staining of Ki-67 and cleaved caspase-3 of MC38 tumour as described in Fig. 5o. The percentage of Ki-67- or cleaved caspase-3-positive cells was quantified (n = 6). Scale bars, 200 μm. Data are presented as means ± s.d., and statistical analyses were performed using a two-tailed unpaired Student’s t-test. Data are representative of three independent experiments. Source numerical data and unprocessed blots are provided in Source Data.
Extended Data Fig. 9 STING restricts aerobic glycolysis and promotes antitumour immunity in established tumours.
a, the weight of the tumours at 14 days post-transplantation as described in Fig. 6b was shown (n = 6 in each group). b, Flow cytometry analysis of tumour-infiltrating lymphocytes in MC38 tumours as described in Fig. 6b (n = 6). c, Flow cytometry analysis of tumour-infiltrating CD4+ T cells and the severe exhausted CD4+ cells in MC38 tumours as described in Fig. 6b (n = 6). d and e, Flow cytometry analysis of CD69+ (d) or CD44+ (e) cells in tumour-infiltrating CD8+ cells in MC38 tumours as described in Fig. 6b (n = 6). f, the weight of the tumours at 16 days post-transplantation as described in Fig. 6g was shown (n = 5). g, Flow cytometry analysis of lymphocytes in MC38 tumours as described in Fig. 6g (n = 5). h and i, Flow cytometry analysis of tumour-infiltrating CD4+ T cells in lymphocytes (h) and the severely exhausted CD4+ T cells (i) in MC38 tumours as described in Fig. 6g (n = 5). j, Measurement of lactate levels in serum, lung, spleen, brain, heart and skeletal muscle of Sting1+/+ and Sting1−/− mice (n = 10 in Sting1+/+ group and n = 7 in Sting1−/− group). k and l, representative figures showing gating strategies for all flow cytometry analysis in this study. Data are presented as means ± s.d., and statistical analyses were performed using a two-tailed unpaired Student’s t-test. Data are representative of three independent experiments. Source numerical data and unprocessed blots are provided in Source Data.
Extended Data Fig. 10 STING restricts aerobic glycolysis in CRC tissues and human CRC cell lines.
a, Representative IHC staining of tumour tissue in human colorectal carcinomas. Scale bar, 200 μm. b, Correlations between the STING levels and the expression of the indicated markers in colorectal tumour tissue (n = 84). c, Correlations between the lactate levels and the expression of the indicated markers in colorectal tumour tissue (n = 84). d, Correlations between the STING or lactate levels and the expression of CA9 in colorectal tumour tissue (n = 84). e, WCLs from HCT116, LoVo and RKO cells were analysed by immunoblotting. f, HCT116 cells were transduced with sgRNA targeting STING to generate knockout cells, while LoVo and RKO cells were transduced to stably express STING. WCLs were analysed by immunoblotting, and lactate levels were quantified (n = 3 biological repeats). Data are presented as means ± s.d., and statistical analyses were performed using a two-tailed unpaired Student’s t-test for f. A two-sided Pearson’s chi-squared test was performed for b–d. Data are representative of three independent experiments. Source numerical data and unprocessed blots are provided in Source Data.
Supplementary information
Supplementary Table 1
Information for MS, primer sequences and antibodies.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Statistical source data for Fig. 6.
Source Data Fig. 7
Statistical source data for Fig. 7.
Source Data Fig. 8
Statistical source data for Fig. 8.
Source Data Fig. 1
Unprocessed western blots for Fig. 1.
Source Data Fig. 2
Unprocessed western blots for Fig. 2.
Source Data Fig. 3
Unprocessed western blots for Fig. 3.
Source Data Fig. 4
Unprocessed western blots for Fig. 4.
Source Data Fig. 5
Unprocessed western blots for Fig. 5.
Source Data Fig. 6
Unprocessed western blots for Fig. 6.
Source Data Fig. 7
Unprocessed western blots for Fig. 7.
Source Data Fig. 8
Unprocessed western blots for Fig. 8.
Source Data Extended Data Fig. 1
Statistical source data for Extended Fig. 1.
Source Data Extended Data Fig. 2
Statistical source data for Extended Fig. 2.
Source Data Extended Data Fig. 3
Statistical source data for Extended Fig. 3.
Source Data Extended Data Fig. 4
Statistical source data for Extended Fig. 4.
Source Data Extended Data Fig. 5
Statistical source data for Extended Fig. 5.
Source Data Extended Data Fig. 6
Statistical source data for Extended Fig. 6.
Source Data Extended Data Fig. 7
Statistical source data for Extended Fig. 7.
Source Data Extended Data Fig. 8
Statistical source data for Extended Fig. 8.
Source Data Extended Data Fig. 9
Statistical source data for Extended Fig. 9.
Source Data Extended Data Fig. 10
Statistical source data for Extended Fig. 10.
Source Data Extended Data Fig. 1
Unprocessed western blots for Extended Fig. 1.
Source Data Extended Data Fig. 2
Unprocessed western blots for Extended Fig. 2.
Source Data Extended Data Fig. 3
Unprocessed western blots for Extended Fig. 3.
Source Data Extended Data Fig. 4
Unprocessed western blots for Extended Fig. 4.
Source Data Extended Data Fig. 5
Unprocessed western blots for Extended Fig. 5.
Source Data Extended Data Fig. 6
Unprocessed western blots for Extended Fig. 6.
Source Data Extended Data Fig. 7
Unprocessed western blots for Extended Fig. 7.
Source Data Extended Data Fig. 10
Unprocessed western blots for Extended Fig. 10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Jiang, C., Zhong, Y. et al. STING is a cell-intrinsic metabolic checkpoint restricting aerobic glycolysis by targeting HK2. Nat Cell Biol 25, 1208–1222 (2023). https://doi.org/10.1038/s41556-023-01185-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01185-x
This article is cited by
-
Scutellarin activates IDH1 to exert antitumor effects in hepatocellular carcinoma progression
Cell Death & Disease (2024)
-
Harnessing innate immune pathways for therapeutic advancement in cancer
Signal Transduction and Targeted Therapy (2024)
-
cGAS-STING pathway mediates activation of dendritic cell sensing of immunogenic tumors
Cellular and Molecular Life Sciences (2024)