Abstract
Significant indirect healthcare costs are related to uncontrolled asthma, including productivity loss. Days with short-acting beta-agonist (SABA) use is associated with symptom-related disruptions at work, home, and school. Digital self-management platforms may support fewer days with SABA medication use and may reduce symptom-related disruptions.
Similar content being viewed by others
Introduction
Poorly controlled asthma, representing ~60% of children and adults with asthma in the United States1, can result in significant healthcare costs and lost productivity at school, work, and home, of which the latter is estimated to cost $3B annually2. A similar loss in productivity is also observed globally3. Digital health solutions have shown promising results in promoting improved clinical outcomes and reducing healthcare resource utilization in asthma4,5, but few studies have explored its potential to assess productivity with passively-collected electronic data.
Electronically-recorded short-acting beta-agonist (SABA) medication use may serve as an important objective determinant of asthma control, as shown in Anderson et al.6 who found that electronically-recorded SABA usage was correlated with self-reported SABA use as captured by question 4 of the Asthma Control Test (ACT). Further, electronic tools like electronic medication monitors (EMMs) could reduce recall bias associated with self-reported symptom surveys like the ACT or the Asthma Control Questionnaire. We hypothesized that electronically-recorded days without use (“SABA-free days”, SFD) may also act as an important objective proxy for productivity. As such, this study aimed to understand the relationship between electronically-captured SFD and self-reported productivity, representing symptom-related disruptions at work, school, and home from question 1 of the ACT, and how these outcomes change over time when enrolled in a digital self-management platform.
Results
The relationship between ACT Q1 and SABA-free days
In the first analysis assessing the relationship between ACT Q1 and SFD, 3,322 adolescents and adults were included (73.8% female, mean (SD) age: 40.7 (13.8) years). Self-reported symptom-related disruption was significantly associated with SFD, with higher disruption observed when fewer SFD were noted (Fig. 1). The lowest symptom-related disruption response (“none of the time” in the ACT) was reported by 1,102 patients for a median (IQR) of 83.9% (61.3, 90.3%) of SFD in the 30 preceding days. 169 patients reported the highest symptom-related disruption response (“all of the time” in the ACT) with a median (IQR) of 51.6% (16.1, 77.4%) of SFD.
Box plots of self-reported productivity and SABA-free days. Box-plot elements include: center line is the median value; the box lower and upper limits correspond to the first and third quartiles; the upper whisker extends from the upper limit to the largest value no further than 1.5 × interquartile range (IQR). The lower whisker extends from the lower limit to the smallest value, at most 1.5 × IQR of the hinge. Data beyond the end of the whiskers are outliers and shown individually.
All of the pairwise comparisons from the Kruskal–Wallis’s Dunn’s post hoc test were significant (p < 0.001), with the exception of “all of the time” and “most of the time” (p = 0.18) (Table 1). Moreover, the association between ACT Q1 and SFD is further confirmed by the adjusted ordinal logistic regression analysis. Specifically, we observed a rise of 2.0% in the odds of increased productivity (i.e., fewer symptom-related disruptions) for every additional SFD (OR 1.02 (95% CI: 1.01, 1.03; p < 0.001), keeping all other factors constant.
Changes in ACT, ACT Q1, and SABA-free days over time
In the second analysis assessing changes in ACT, ACT Q1, and SFD over 90 days, 1595 adolescents and adults were included (75.1% female, mean (SD) age: 41.5 (13.8) years, 86.6% uncontrolled asthma at enrollment (ACT≤19)). From enrollment to follow-up, the percentage of SFD increased by 10% (median IQR: 70% (43, 87%) vs. 80% (57, 90%); p < 0.001). Similarly, symptom-related disruptions improved across all responses to ACT Q1 (all p < 0.01) from enrollment to follow-up. Total ACT score also improved (median (IQR): 14 (11, 17) vs. 17 (13, 21); p < 0.001), with 46.3% of patients demonstrating a clinically meaningful improvement, defined as ≥3 point increase in total ACT score (Table 2).
Discussion
This study observed that electronically-captured SABA-free days were associated with symptom-related disruptions at work, school, and home. Further, the use of a digital platform was associated with a significant improvement in SFD and a reduction in monthly self-reported symptom-related disruptions in adolescents and adults with asthma.
Such insights may be important, especially when considering the indirect costs of uncontrolled asthma7,8,9. In the US, the cost of missed days of school and work for people with asthma is estimated to be $3B/year2. In our study, we observed that over 90 days, patients had a 10% increase in SFD (or nine SFDs (10% increase × 90 days)) and a 2% reduction in symptom-related disruptions associated with each additional SFD. As such, we calculated an 18% reduction (2% × nine more SFDs) in symptom-related disruptions, which could be crudely translated to a yearly cost savings of $540 M ($3B/year × 18%) assuming a constant rate in the reduction in disruptions.
Several studies have already demonstrated that the use of digital tools can also support asthma management, mainly by increasing adherence to treatment, identifying therapeutic failure to intervene early, or informing therapeutic adjustments4,5,6,10. The COVID-19 pandemic has further highlighted the value of remote monitoring, where connected physiologic or therapeutic monitoring can be used to support patient care when in-person clinic visits are not always feasible.11 While barriers to remote care adoption still exist among patients and healthcare providers, the pandemic has presented an opportunity to address these constraints and refine current digital health approaches to better support the current standard of care in asthma.
The present study demonstrates the value of electronically-captured medication use, but there are several limitations to consider. First, patients self-reported their history of asthma physician confirmation was not sought. Patients may also have used multiple inhalers, not all of which had an EMM, thus possibly preventing the assessment of complete medication usage. ACT Q1 is a self-reported measure and relies on perceived days of symptom-related disruptions and should be confirmed with additional productivity data. Further, patients included in these analyses may have been more motivated, possibly overestimating the associations observed. Future studies should explore the present findings in a larger sample size, confirm findings in more robust study designs that include a comparison group, and consider the impact of controller medication use and disease severity subgroups.
SFD may be an important objective proxy for perceived symptom-related disruptions, allowing for more regular quantification of disease impact compared to self-reported measures, which may not be completed regularly and face self-report biases. We also observed the continued clinical benefit of digital health platforms, with improvements in asthma control (ACT), productivity (ACT Q1), and SFD over the study period. Digital platforms may provide important insights into patient medication use, which can be used to enhance patient care.
Methods
Study design
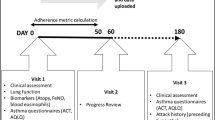
This analysis used retrospective data collected from patients aged ≥12 years with a self-reported history of asthma who utilized a digital self-management platform (Propeller Health, Madison, WI, USA)5 between 2016 and 2019. Patients self-enrolled at health fairs and via social media campaigns or were recruited through clinical programs offered at their healthcare organizations. Patients did not receive monetary compensation for their participation.
Data collection
To set up the digital platform, patients attached an EMM to their SABA inhaler to passively record the date and time of each actuation. The days in which the patient did not use their SABA medication were classified as SFD. Patients also paired their EMM with a companion smartphone app, which helped patients track medication usage and trends and provided evidence-based educational content5.
At enrollment and every month thereafter, patients were prompted to complete an Asthma Control Test (ACT) in the app to assess their own perception of daily functioning, symptom-related disruption, and symptom control in the four preceding weeks12. The first question of the ACT (ACT Q1) focuses on self-reported symptom-related disruptions, asking, “In the past 4 weeks, how much of the time did your asthma keep you from getting as much done at work, school, or at home?”. Responses range from 1 (all of the time) to 5 (none of the time).
Ethics approval
All patients agreed to Propeller’s Terms of Use, which allows for retrospective analysis of de-identified aggregate data. The current retrospective analysis proposal was determined to be exempt by the Copernicus Institutional Review Board (PRH1-18-132).
Retrospective analysis
The relationship between ACT Q1 and SFD was assessed cross-sectionally in patients with ≥30 days of continuous EMM data, which captured SABA use preceding a completed ACT. The Kruskal–Wallis test with Dunn’s post hoc comparison test was used to determine if the percent of SFDs in the 30 days preceding an ACT statistically differed between the five response options of ACT Q1 (all of the time, most of the time, some of the time, a little of the time, none of the time). To account for the multiple statistical tests performed simultaneously, Bonferroni correction was applied for Dunn’s post hoc comparison. We further explored the association between ACT Q1 and SFD by performing an ordinal logistic regression model adjusting for age, gender, baseline ACT, season, enrollment site, and controller inhaler use.
The change in total ACT score, ACT Q1, and SFD over time was evaluated between enrollment and month 3 (days 68–97). Patients needed to have ≥90 days of continuous EMM data, ≥1 EMM-recorded SABA usage, and 2 completed ACTs (at baseline and 3 months) to be included in the analysis. Changes over time in the total ACT and SFD were assessed using Wilcoxon signed-rank test for paired data (e.g., consisting of repeated measurements). For the changes over time in ACT Q1, the Stuart-Maxwell test checked the overall difference of all five response options to ACT Q1 at baseline and at month 3. After that, McNemar’s tests were used to test if there is a statistically significant change between baseline and month 3 in each level.
All statistical tests were two-tailed with an alpha = 0.05 threshold for statistical significance, except for Dunn’s post hoc comparison test (alpha = 0.005). All analyses were conducted in R version 4.1.1 (R Foundation for Statistical Computing).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
De-identified data are available upon request with appropriate regulatory and third-party authorization.
Code availability
Code will be made available upon request.
References
CDC. Uncontrolled asthma among adults, 2016. https://www.cdc.gov/asthma/asthma_stats/uncontrolled-asthma-adults.htm.
Nurmagambetov, T., Kuwahara, R. & Garbe, P. The economic burden of asthma in the United States, 2008–2013. Ann. Am. Thorac. Soc. 15, 348–356 (2018).
Dierick, B. J. et al. Work absence in patients with asthma and/or COPD: a population-based study. NPJ Prim. Care Resp. Med. 31, 1–7 (2021).
Lin, N. Y. et al. Telehealth delivery of adherence and medication management system improves outcomes in inner- city children with asthma. Pediatr. Pulmonol. 55, 858–865 (2020).
Mosnaim, G. S. et al. The impact of patient self-monitoring via electronic medication monitor and mobile app plus remote clinician feedback on adherence to inhaled corticosteroids: a randomized controlled trial. J. Allergy Clin. Immunol. Pract. 9, 1586–1594 (2021).
Anderson, W. C. III et al. Assessing asthma control: comparison of electronic-recorded short-acting beta-agonist rescue use and self-reported use utilizing the asthma control test. J. Asthma 58, 271–275 (2021).
Rappaport, H. & Bonthapally, V. The direct expenditures and indirect costs associated with treating asthma in the United States. J. Aller. Ther. 3, 118 (2012).
Yaghoubi, M., Adibi, A., Safari, A., FitzGerald, J. M. & Sadatsafavi, M. The projected economic and health burden of uncontrolled asthma in the United States. Am. J. Respir. Crit. Care Med. 200, 1102–1112 (2019).
Song, H. J., Blake, K. V., Wilson, D. L., Winterstein, A. G. & Park, H. Medical costs and productivity loss due to mild, moderate, and severe asthma in the United States. J. Asthma Allergy 13, 545–555 (2020).
Chan, A. H. Y. et al. Effect of electronic adherence monitoring on adherence and outcomes in chronic conditions: a systematic review and meta-analysis. PLoS ONE 17, e0265715 (2022).
Mosnaim, G. S., Stempel, H., Van Sickle, D. & Stempel, D. A. The adoption and implementation of digital health care in the post–COVID-19 era. J. Allergy Clin. Immunol. Pract. 8, 2484–2486 (2020).
Nathan, R. A. et al. Development of the asthma control test: a survey for assessing asthma control. J. Allergy Clin. Immunol. 113, 59–65 (2004).
Acknowledgements
This study was funded by Propeller Health and ResMed Science Center. With thanks to Vanessa Di Cataldo, Ph.D. of ResMed Science Center, for her technical writing support.
Author information
Authors and Affiliations
Contributions
Study design, manuscript writing, and commenting on manuscript and approval manuscript: T.G., L.K., M.A.B., E.B., and V.V. Data collection: M.A.B., L.K., and V.V. Data analysis: V.V.
Corresponding author
Ethics declarations
Competing interests
L.K., M.A.B., V.V., and E.B. are employees of ResMed, the parent company of Propeller Health. L.K. and M.A.B. are former employees of Propeller Health. T.G. reports personal fees from the American Board of Pediatrics; Pediatric Pulmonary Subboard, grants from NIH, grants and personal fees from Sanofi/Regeneron/Amgen, grants and personal fees from AstraZeneca, personal fees from Novartis, personal fees from GSK, personal fees from TEVA, royalties from UpToDate.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaye, L., Vuong, V., Barrett, M.A. et al. Improvement in symptom-related disruptions is associated with fewer days of short-acting beta-agonist use in asthma. npj Prim. Care Respir. Med. 32, 31 (2022). https://doi.org/10.1038/s41533-022-00299-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-022-00299-3