Abstract
While overweight/obesity has become a major public health issue worldwide, any association between body mass index (BMI) and therapeutic response in neoadjuvant targeted therapy treated HER2 positive breast cancer patients remain unclear. The information from a total of four-hundred and ninety-one neoadjuvant targeted therapy treated HER2 positive breast cancer patients from four institutions were retrospectively collected. Univariate and multivariate logistic analysis was developed to determine the association between BMI and therapeutic response. A meta-analysis of published literature was then conducted to confirm the effect of overweight/obesity on pCR for patients treated with neoadjuvant targeted therapy. Restricted cubic spline (RCS) adjusted for confounding factors demonstrated a decrease pCR with increasing BMI (OR = 0.937, P = 0.045). Patients were then categorized into under/normal weight (n = 299) and overweight/obesity (n = 192). Overweight/obese patients were independently associated with a poor therapeutic response. In the subgroup analysis, a significant negative impact of overweight/obesity on pCR can be observed both in single-targeted (OR = 0.556; P = 0.02) and dual-targeted (OR = 0.392; P = 0.021) populations. Six eligible studies involving 984 neoadjuvant targeted therapy treated HER2 positive breast cancer patients were included in the meta-analysis. The meta-analysis also demonstrated that overweight/obesity was significantly associated with a poor response to neoadjuvant anti-HER2 therapy (OR = 0.68; P = 0.007). Our result show that overweight and obese HER2 positive breast cancer patients are less likely to achieve pCR after neoadjuvant targeted therapy.
Similar content being viewed by others
Introduction
Overweight/Obesity is conventionally defined by the body mass index (BMI), characterized by abnormal or excessive accumulation of body fat, and has become a major public health issue worldwide1. It is widely accepted that overweight/obesity is associated with the development of breast cancer, as well as influencing its prognosis, particularly in hormone receptor (HR) positive postmenopausal breast cancer2,3,4. Although the underlying mechanism remains obscure, several studies have proposed that the chronic inflammatory state, circulating level of adipokines, insulin, insulin-like growth factor (IGF), and sex hormones might mediate the connection between overweight/obesity and breast cancer5.
Neoadjuvant therapy is increasingly used for treating patients with breast cancer6. A pathologic complete response (pCR) after neoadjuvant therapy can serve as an indicator of individual long-term survival7. Exploring the impact of BMI on pCR after neoadjuvant therapy provides an opportunity to assess whether BMI affects the therapeutic response in vivo, and more importantly, it might be informative for the association between BMI and long-term prognosis in breast cancer8. Irrespective of the potential mechanisms, there have been numerous studies committed to unraveling the relationship between BMI and the neoadjuvant therapeutic response. A recently published meta-analysis reports a negative impact of high BMI on pCR8. Whereas some studies suggest no association, or even demonstrate a positive effect of obesity on the pCR9,10. Notably, these studies included all molecular subtypes of breast cancer, which might lead to paradoxical conclusions.
Among HER2 positive patients, exploratory analysis of the NeoALTTO trial have demonstrated a borderline significant decreased pCR rate for overweight/obese patients with HR + /HER2+ breast cancer (OR = 0.55, P = 0.053), but not in HR-/HER2+ breast cancer (OR = 1.3, P = 0.331)11. While the subgroup analysis from a pooled study of eight prospective trials conducted by Fontanella et al. reported no association between BMI and pCR among HER2 positive breast cancer patients12. Currently, chemotherapy plus trastuzumab ± pertuzumab has become the standard preoperative therapy for high risk HER2 positive breast cancer. However, few studies have specifically focused on this population, rendering an unmet need to reveal the impact of BMI on pCR for HER2 positive patients treated with standard neoadjuvant therapy.
Herein, we report a retrospective multi-center analysis to determine the impact of BMI on the response to neoadjuvant targeted therapy for HER2 positive breast cancer. A meta-analysis of published studies was carried out to further validate the association between overweight/obesity and pCR for patients treated with neoadjuvant targeted therapy.
Results
Baseline characteristics
A total of 491 patients with a median BMI of 23.04 were included in our study. Their baseline characteristics arranged by BMI category are listed in Table 1. Among these patients, 299 (60.9%) patients were categorized into the under/normal weight group, while 192 (39.1%) cases were classified into overweight/obesity. Overweight/obesity was associated with a higher age. A significant correlation between BMI category and pCR was also observed (P = 0.008). No significant association was found between the BMI category and other factors.
BMI is associated with therapeutic response as a continuous or categorical variable
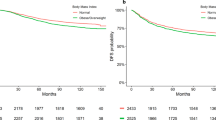
Overall, 189 (38.5%) patients achieved a pCR after neoadjuvant targeted therapy. A restricted cubic spline with 3-knots was developed to account for the impact of BMI on the neoadjuvant response as a continuous variable. After adjusting for potential confounders (P < 0.1 in univariate analysis) (Supplementary Table 1), RCS showed that a patient is less likely to achieve a pCR as the BMI increases (Fig. 1). Indeed, BMI was significantly associated with pCR as a continuous variable after adjusted by confounding factors (OR = 0.937, P = 0.045; data not shown).
The association between the BMI and the therapeutic response was further investigated by using BMI as a categorical variable. The pCR rates of the under/normal weight, overweight/obesity groups were 43 and 31%, respectively. Patients in the overweight/obese groups were independently associated with a poor pCR rate compared to the under-/normal weight groups in multivariate analysis (OR = 0.497, P = 0.001; Table 2). Collectively, our analysis suggests that BMI is correlated with therapeutic response in neoadjuvant targeted therapy treated HER2 positive breast cancer patients.
Association between overweight/obesity and pCR in subgroup analysis
A subgroup analysis was conducted to further explored the implication of BMI in HER2 positive breast cancer patients. As shown in Fig. 2, overweight/obesity was independently associated with a poor response to neoadjuvant targeted therapy despite the patient’s age, tumor size, nodal status, hormone receptor status, Ki67 expression, or neoadjuvant targeted therapy. We also observed a statistically significant lower pCR with the overweight/obesity group for the premenopausal and HER2 3+ populations. Overweight/obese patients are less likely to achieve pCR among postmenopausal and HER2 2+ populations, although in our study this did not reach statistical significance. Overall, our subgroup analysis demonstrated a consistent trend as in the main analysis.
Meta-analysis of the implication of overweight/obesity on pCR for HER2 positive breast cancer
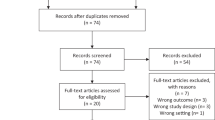
The literature search identified 32 studies eligible for full text review. Only six studies met the inclusion criteria and were included in our meta-analysis. Among these studies only one study was derived from a prospective randomized clinical trial11, the remaining five studies were retrospective13,14,15,16,17. The flowchart of our literature search and the characteristics of eligible studies are shown in Fig. 3 and Table 3.
All six studies provided data that divided patients into two groups (BMI < 25 kg/m2 and ≥25 kg/m2), hence, our pooled analysis investigated the impact of overweight/obesity (BMI ≥ 25 kg/m2) on the neoadjuvant therapy response versus under/normal weight patients (BMI < 25 kg/m2). A total of 984 HER2 positive breast cancer patients treated with anti-HER2 based neoadjuvant therapy from six studies were included in our meta-analysis. We found a significant decrease in the pCR in overweight/obese patients compared to the under/normal weight patients (OR = 0.68, P = 0.007; Fig. 4), consistent with our results.
Discussion
In the present study, we surveyed data from 491 patients and found that overweight/obesity was associated with a lower pCR rate in HER2 positive breast cancer patients after neoadjuvant targeted therapy. In addition, a meta-analysis of published literature was conducted, and it further confirmed the negative influence of BMI on the pCR in a targeted therapy treated HER2+ breast cancer population.
Substantial efforts have been made to investigate the influence of BMI on the therapeutic response of breast cancer patients, but there remains controversy. A recently published meta-analysis revealed that overweight/obese patients had a lower pCR rate compared to under/normal weight patients8. This result was consistent with several studies18,19. However, some studies have offered conflicting perspectives9,20, but these studies did not focus on a specific subtype of breast cancer. Breast cancer is a heterogeneous disease with different subtypes and the mechanism of carcinogenesis, and the treatment strategies vary among these subtypes21. The BMI may play a diverse role in the neoadjuvant therapeutic response depending on the subtype of breast cancer, and this might explain the conflicting results from previous studies.
In our study, we found an independent negative impact of BMI on pCR in HER2 positive breast cancer patients treated with neoadjuvant targeted therapy. The underlying molecular mechanisms by which overweight/obesity could influence the effect of targeted therapy are currently unclear. Sex hormones have been reported to mediate cross talk between overweight/obesity and breast cancer due to the endocrine role of adipose tissue, especially in postmenopausal women, even though BMI does not measure adiposity directly4,5,22,23. Indeed, analysis of the NeoALTTO trial shows a tendency towards a lower pCR rate for the overweight/obesity patients compared to the under/normal weight patients in HR + /HER2+ (OR = 0.56, P = 0.054), but not in HR-/HER2+ (OR = 1.31, P = 0.324) breast cancer patients11. Interestingly, a similar effect of overweight/obesity on pCR was demonstrated in HR-/HER2+ (OR = 0.522, P = 0.025) and HR + /HER2+ (OR = 0.525, P = 0.049) breast cancer patients in our analysis. We also observed a significant negative impact of BMI on pCR in premenopausal patients. This suggests that other mechanisms rather than sex hormones are involved.
The tumor immune microenvironment (TIME) plays an important role in the therapeutic response in HER2 positive breast cancer. The impact of tumor immunity is important for patients treated with targeted therapy since the effect of trastuzumab and pertuzumab is partially reliant on antibody-dependent cellular cytotoxicity (ADCC)24. It has been reported that adiposity accumulation can result in an immunosuppressive TIME25. For instance, overweight/obesity-related increases in the chemokine CCL2, estrogen, and pro-inflammatory mediators could induce the accumulation of myeloid-derived suppressor cells (MDSCs) in breast cancer tumors11,26. In addition, a lower density of tumor-infiltrating lymphocytes (TILs) has been reported in overweight/obese HER2+ breast cancer patient tumors compared to tumors from normal weight counterparts17. The immunosuppressive status of the TIME might contribute to the poor targeted therapy response in overweight/obese HER2 positive breast cancer patients.
In vitro and in vivo studies have suggested that adipose cells promote the resistance to trastuzumab-mediated ADCC via the secretion of soluble factors, despite the mechanism remaining unclear27. The immunological function of leptin has recently attracted much interest, especially for the impact of leptin on natural killer (NK) cells – one of the most crucial mediators of ADCC28,29. A short-term stimulation of leptin could result in a significant NK cell functional activation. However, chronic exposure to an elevated concentration of leptin in overweight/obesity might decrease the anti-tumor immune response of NK cell by inhibiting NK cell functions30. In addition to the immunological role of leptin, the crosstalk between leptin signaling and HER2 signaling might also reduce sensitivity to targeted therapy, thus lead to a low pCR rate31.
Overweight/obese patients tend to have an increased concentration of circulating insulin-like growth factors-1 (IGF-1) that can result in an overactivation of insulin-like growth factors-1 receptor (IGF-1R) signaling32,33. Preclinical studies have revealed that the aberrant activation of IGF-1R signaling promotes cell survival via the PI3K-AKT-mTOR and RAS-RAF-MAPK pathways and contribute to the poor response to anti-HER2 therapy34. The association between circulating IGF-1 and pCR has been investigated by Tong et al. in a retrospective clinical study, where they found that a low IGF-1 level was related with a higher pCR rate (OR = 3.93, P = 0.031) in trastuzumab treated HER2 positive breast cancer patients14. Therefore, elevated circulating IGF-1 levels in overweight/obese patients might contribute to the inferior targeted therapy response in this population.
The pharmacokinetics of monoclonal antibodies associated with obesity might also be involved in the poor response to targeted therapy. It has been demonstrated that higher body weight of patients is associated with increased trastuzumab or pertuzumab clearance, which might lead to a lower plasma concentration in overweight/obese patients35,36. It is hard to determine the relationship between the trastuzumab plasma concentration and neoadjuvant therapeutic response due to the lack of relevant data. However, a lower trastuzumab concentration has been observed in metastatic gastric cancer patients with progressive disease than in those with partial or complete response or stable disease37. These findings might bring new insights, although it might not be appropriate to extrapolate the conclusion from gastric cancer to breast cancer38.
Our study has several limitations. The first limitation is the retrospective nature of the study. Secondly, Asians at a given BMI have a higher percentage of body fat than White or European populations39. Due to the variation in the body composition across regions and ethnicities, we defined overweight/obesity according to the Chinese WGOC definition in our multi-center study, since it was derived from a large Chinese population40. However, WHO criteria were employed in the subsequent meta-analysis. The differences of definition in overweight/obesity between multi-center study and meta-analysis might lead to a difficulty in the interpretation of our research. Finally, we could not explore the association between BMI and the prognosis of HER2+ patients due to the lack of prognostic information.
In conclusion, based on a multi-center retrospective study and meta-analysis, we found a negative impact of overweight/obesity on the therapeutic response for HER2 positive breast cancer patients treated with neoadjuvant targeted therapy. Further studies are needed to shed light on the complex mechanism behind this phenomenon.
Methods
Patient selection and data acquisition
Information from a total of 491 patients diagnosed with HER2 positive breast cancer, all of whom had undergone neoadjuvant targeted therapy followed by surgery between June 2012 to December 2021, was consecutively collected from four institutions (Fujian Medical University Union Hospital, Fujian Cancer Hospital, Zhangzhou Affiliated Hospital of Fujian Medical University, and No. 900 Hospital of The Joint Logistic Support Force). Breast cancer diagnosis was confirmed by core needle biopsy prior to neoadjuvant therapy. Neoadjuvant regimens consisted of: 1) EC-T plus targeted therapy (epirubicin 100 mg/m2 and cyclophosphamide 600 mg/m2 every three weeks for four cycles followed by docetaxel 80 mg/m2 and targeted therapy every three weeks for four cycles), 2) TCb plus targeted therapy (docetaxel 75 mg/m2, carboplatin [area under curve = 6] and targeted therapy every three weeks for six cycles), 3) THP (docetaxel 80 mg/m2, trastuzumab initiated with a loading dose of 8 mg/kg followed by a maintenance dose of 6 mg/kg, and pertuzumab 840 mg as a loading dose at cycle 1 and 420 mg thereafter every three weeks for four cycles). Trastuzumab or trastuzumab plus pertuzumab was given for patients who received targeted therapy. Patients with dose reduction were excluded from the present study.
Clinicopathological characteristics of participants were collected, including demographic information, imaging examination, pathological evaluation of biopsy and surgical specimens, and treatment records. A median age of 49 was employed to classify patients into two groups. Women were considered postmenopausal if they had no menstruation in the past 12 months. Clinical tumor size and axillary lymph node status before neoadjuvant therapy was evaluated by color Doppler ultrasound and was defined in accordance with American Joint Committee on Cancer (AJCC) breast cancer staging manual 8th. Patients with metastatic disease, and those with unavailable baseline BMI were excluded from the study. The protocol of this study was approved by the ethics committee of Fujian Medical University Union Hospital, ethics committee of Fujian Cancer Hospital, ethics committee of Zhangzhou Affiliated Hospital of Fujian Medical University, and ethics committee of No. 900 Hospital of The Joint Logistic Support Force. All participants gave their written informed consent before their inclusion.
BMI calculation and categorization
The height and weight of patients was recorded within one week prior to receiving neoadjuvant therapy in their first hospitalization. BMI was calculated as the weight (Kg) divided by the square of the height (m2) and was used to defined obesity in our study. Due to the variation in the body composition across regions and ethnicities, the WGOC definition derived from a large Chinese population was employed to categorize patients into underweight (BMI < 18.5 kg/m2), normal weight (18.5 to <24 kg/m2), overweight (24 to <28 kg/m2), and obese (≥28 kg/mg2)39,40. The impact of BMI on the neoadjuvant therapeutic response was investigated as a continuous or categorical variable.
Pathological evaluation
Estrogen receptor (ER), progesterone receptor (PR), and HER2 were evaluated by immunohistochemistry (IHC). Patients were defined as positive for ER or PR if at least 1% of the tumor nuclei were stained, and they were hormone receptor (HR) negative when both ER and PR were found to be negative. HER2 was defined as positive when IHC results were 3+ or 2+ with HER2 amplification evaluated by fluorescence in situ hybridization (FISH)41. A median Ki67 index (by IHC) of 40% was used to divide patients into a low Ki67 index group (<40%) and a high Ki67 index group (≥40%). A pCR was defined as the absence of residual invasive disease in the breast and axillary lymph nodes (ypT0/is, ypN0).
Literature review and meta-analysis
A systematic literature search and meta-analysis was performed to further investigate the implications of BMI on neoadjuvant targeted therapy of HER2 positive breast cancer patients. Relevant research published prior to December 1, 2021, was retrieved from Pubmed, Embase, Web of Science, and Google Scholar. The key search terms were “breast neoplasms” or “breast cancer”, “neoadjuvant therapy” or “neoadjuvant” or “pathologic complete response”, “trastuzumab” or “molecular targeted therapy”, “body mass index” or “obesity” or “overweight”. Literature retrieval was also conducted by reviewing the references of reviewed studies. Studies were eligible for inclusion if they; (1) had available data of the distribution of the BMI category and corresponding pCR rate in HER2 positive breast cancer, (2) neoadjuvant targeted therapy was given for HER2 positive breast cancer, and (3) pCR was defined as no residual invasive cancer in breast or nodes with residual noninvasive breast cancer allowed (ypT0/is ypN0), or the absence of invasive cancer cells in the breast irrespective of the presence of lymph node infiltration by malignant cells (ypT0/is). Two authors independently performed the literature search, study screening, and data extraction.
Statistical analysis
A chi-square test was employed to explore the association between the BMI categories and the following clinicopathological characteristics: age, menopausal status, clinical T stage, Nodal status, hormone receptor status, HER2 staining intensity, Ki67, neoadjuvant targeted therapy, and pathological response. An RCS with 3-knots was used to allow the investigation of a non-linear association between BMI and pCR. Univariate logistic analysis was used to assess factors correlated with the pCR, factors with a P < 0.1 in univariate analysis were included in multivariate analysis (menopausal status was excluded from multivariate analysis due to the high consistency between menopausal status and age). All statistical analyses were performed by R (version 4.0.0) and SPSS (Version 26.0).
The meta-analysis was performed by Review Manager 5.4. Heterogeneity was assessed by I2 statistics, and an I2 < 50% indicated there was no heterogeneity among these studies, and a fixed effect model was used in meta-analysis. Otherwise, a random effect model was applied. We conducted a funnel plot to assess publication bias. If the funnel plot was symmetric, then there was no publication bias. P values were significant at P < 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data were available from corresponding authors upon reasonable request.
References
GBD 2015 Obesity Collaboratorset al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 67, 378–397 (2017).
Calle, E. E. & Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004).
Klintman, M. et al. Postmenopausal overweight and breast cancer risk; results from the KARMA cohort. Breast Cancer Res. Treat. 196, 185–196 (2022).
Zhao, C. et al. Current landscape: the mechanism and therapeutic impact of obesity for breast cancer. Front Oncol. 11, 704893 (2021).
Miglietta, F., Dieci, M. V., Griguolo, G. & Guarneri, V. Neoadjuvant approach as a platform for treatment personalization: focus on HER2-positive and triple-negative breast cancer. Cancer Treat. Rev. 98, 102222 (2021).
Yau, C. et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 23, 149–160 (2022).
Wang, H. et al. Impact of body mass index on pathological complete response following neoadjuvant chemotherapy in operable breast cancer: a meta-analysis. Breast Cancer 28, 618–629 (2021).
Farr, A. et al. The effect of obesity on pathological complete response and survival in breast cancer patients receiving uncapped doses of neoadjuvant anthracycline-taxane-based chemotherapy. Breast 33, 153–158 (2017).
Warner, E. T. et al. Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective Alliance clinical trials (A151426). Breast Cancer Res. Treat. 159, 109–118 (2016).
Di Cosimo, S. et al. Effect of body mass index on response to neo-adjuvant therapy in HER2-positive breast cancer: an exploratory analysis of the NeoALTTO trial. Breast Cancer Res. 22, 115 (2020).
Fontanella, C. et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res. Treat. 150, 127–139 (2015).
Howell, S. J., Coe, F., Wang, X., Horsley, L. & Ekholm, M. Carboplatin dose capping affects pCR rate in HER2-positive breast cancer patients treated with neoadjuvant Docetaxel, Carboplatin, Trastuzumab, Pertuzumab (TCHP). Breast Cancer Res. Treat. 184, 481–489 (2020).
Tong, Y. W. et al. Insulin-like growth factor-1, metabolic abnormalities, and pathological complete remission rate in HER2-positive breast cancer patients receiving neoadjuvant therapy. Onco Targets Ther. 12, 3977–3989 (2019).
Ding, J., Yang, Y., Jiang, L., Wu, W. & Shao, Z. Predictive factors of pathologic complete response in HER2-positive and axillary lymph node positive breast cancer after neoadjuvant paclitaxel, carboplatin plus with trastuzumab. Oncotarget 8, 56626–56634 (2017).
Hamy, A. S. et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann. Oncol. 28, 2233–2240 (2017).
Takada, K. et al. Clinical verification of body mass index and tumor immune response in patients with breast cancer receiving preoperative chemotherapy. BMC Cancer 21, 1129 (2021).
Karatas, F. et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast 32, 237–244 (2017).
Chen, S. et al. Obesity or overweight is associated with worse pathological response to neoadjuvant chemotherapy among Chinese women with breast cancer. PLoS One 7, e41380 (2012).
Erbes, T. et al. BMI and pathologic complete response to neoadjuvant chemotherapy in breast cancer: a study and meta-analysis. Clin. Breast Cancer 16, e119–e132 (2016).
Loibl, S., Poortmans, P., Morrow, M., Denkert, C. & Curigliano, G. Breast cancer. Lancet 397, 1750–1769 (2021).
Carmichael, A. R. & Bates, T. Obesity and breast cancer: a review of the literature. Breast 13, 85–92 (2004).
Prentice, A. M. & Jebb, S. A. Beyond body mass index. Obes. Rev. 2, 141–147 (2001).
Scheuer, W. et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 69, 9330–9336 (2009).
Sanchez-Pino, M. D., Gilmore, L. A., Ochoa, A. C. & Brown, J. C. Obesity-associated myeloid immunosuppressive cells, key players in cancer risk and response to immunotherapy. Obesity 29, 944–953 (2021).
Ostrand-Rosenberg, S. Myeloid derived-suppressor cells: their role in cancer and obesity. Curr. Opin. Immunol. 51, 68–75 (2018).
Duong, M. N. et al. Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res. 17, 57 (2015).
Caligiuri, M. A. Human natural killer cells. Blood 112, 461–469 (2008).
Delort, L., Rossary, A., Farges, M. C., Vasson, M. P. & Caldefie-Chezet, F. Leptin, adipocytes and breast cancer: focus on inflammation and anti-tumor immunity. Life Sci. 140, 37–48 (2015).
Wrann, C. D. et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am. J. Physiol. Endocrinol. Metab. 302, E108–E116 (2012).
Fiorio, E. et al. Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer 8, 305 (2008).
Heetun, A., Cutress, R. I. & Copson, E. R. Early breast cancer: why does obesity affect prognosis? Proc. Nutr. Soc. 77, 369–381 (2018).
Ligorio, F. et al. Prognostic impact of body mass index (BMI) in HER2+ breast cancer treated with anti-HER2 therapies: from preclinical rationale to clinical implications. Ther. Adv. Med. Oncol. 14, 17588359221079123 (2022).
Lu, Y., Zi, X., Zhao, Y., Mascarenhas, D. & Pollak, M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J. Natl Cancer Inst. 93, 1852–1857 (2001).
Bajaj, G., Suryawanshi, S., Roy, A. & Gupta, M. Evaluation of covariate effects on pharmacokinetics of monoclonal antibodies in oncology. Br. J. Clin. Pharmacol. 85, 2045–2058 (2019).
Garg, A. et al. Population pharmacokinetic and covariate analysis of pertuzumab, a HER2-targeted monoclonal antibody, and evaluation of a fixed, non-weight-based dose in patients with a variety of solid tumors. Cancer Chemother. Pharmacol. 74, 819–829 (2014).
Cosson, V. F., Ng, V. W., Lehle, M. & Lum, B. L. Population pharmacokinetics and exposure-response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother. Pharmacol. 73, 737–747 (2014).
Kolberg, H. C. et al. Is weight-based IV dosing of trastuzumab preferable to SC fixed-dose in some patients? A systematic scoping review. Breast 57, 95–103 (2021).
Deurenberg, P., Deurenberg-Yap, M. & Guricci, S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes. Rev. 3, 141–146 (2002).
Zhou, B. F., Cooperative Meta-Analysis Group of the Working Group on Obesity in, C. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 15, 83–96 (2002).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. Arch. Pathol. Lab. Med. 142, 1364–1382 (2018).
Acknowledgements
We thank the patients who participated in this study, and we acknowledge the assistance of Jinxing Lv, Dehui Chen, Jiafan Yu, and Yihua Zhang. This study was supported by grants from Joint Funds for the Innovation of Science and Technology, Fujian Province (2018Y9055, 2019Y9103, and 2020Y9053), Joint Key Funds for the Health and Education of Fujian Province (2019-WJ-23), Fujian Provincial Health Technology Project (2020QNA039), Natural Science Foundation of Fujian Province (2021J01737) and QIHANG Funds of Fujian Medical University (No. 2022QH2025).
Author information
Authors and Affiliations
Contributions
F.F., C.W., X.W., and Y.L. conceived and designed the study, they are co-corresponding authors. Y.L., Q.N., H.C., W.Z., X.J., and Q.C. collected the data. W.G., L.C., and M.C. analyzed the data. F.W. and X.C. performed the literature search, study screening, and data extraction independently. L.W. assisted with pathologic evaluation. L.C., F.W., X.C., Y.C., and L.D. wrote the manuscript, these authors contribute equally to this work, they are co-first authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, L., Wu, F., Chen, X. et al. Impact of body mass index in therapeutic response for HER2 positive breast cancer treated with neoadjuvant targeted therapy: a multi-center study and meta-analysis. npj Breast Cancer 9, 46 (2023). https://doi.org/10.1038/s41523-023-00552-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-023-00552-z