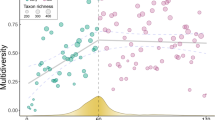
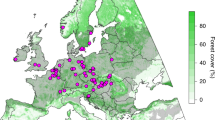
Abstract
The mechanisms underlying plant succession remain highly debated. Due to the local scope of most studies, we lack a global quantification of the relative importance of species addition ‘versus’ replacement. We assessed the role of these processes in the variation (β-diversity) of plant communities colonizing the forelands of 46 retreating glaciers worldwide, using both environmental DNA and traditional surveys. Our findings indicate that addition and replacement concur in determining community changes in deglaciated sites, but their relative importance varied over time. Taxa addition dominated immediately after glacier retreat, as expected in harsh environments, while replacement became more important for late-successional communities. These changes were aligned with total β-diversity changes, which were more pronounced between early-successional communities than between late-successional communities (>50 yr since glacier retreat). Despite the complexity of community assembly during plant succession, the observed global pattern suggests a generalized shift from the dominance of facilitation and/or stochastic processes in early-successional communities to a predominance of competition later on.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw sequence data (Sper01 marker) generated using the protocols described in the ‘Methods’ section are deposited in the ‘Sper01_raw_sequences.zip’ folder available in Zenodo (https://zenodo.org/record/6620359#.Y8E1OP6ZO5d)95. The data that support the findings of this study are provided as Supplementary Tables 1–3.
Code availability
Scripts for reproducing the results in this study are available as Supplementary codes.
Supplementary Code 1. Code reproducing bioinformatics steps.
Supplementary Code 2. Code reproducing taxonomic assignation.
Supplementary Code 3. R code for the MOTU filtering after bioinformatic analyses to remove sequences with best identity <90% and detected at a low frequency that can be artefacts produced by PCR, contaminants and sequencing errors.
Supplementary Code 4. R code to calculate beta-diversity and its components, run the main models and illustrate results.
Supplementary Code 5. R code to test the ability of our sampling design to detect breakpoints in segmented regressions.
References
Connell, J. H. & Slatyer, R. O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 111, 1119–1144 (1977).
Prach, K. & Walker, L. R. Comparative Plant Succession among Terrestrial Biomes of the World (Cambridge Univ. Press, 2020).
Walker, L. R. & del Moral, R. Primary Succession and Ecosystem Rehabilitation (Cambridge Univ. Press, 2003).
Clements, F. E. Plant Succession: An Analysis of the Development of Vegetation (Carnegie Institution of Washington, 1916).
Pulsford, S. A., Lindenmayer, D. B. & Driscoll, D. A. A succession of theories: purging redundancy from disturbance theory. Biol. Rev. 91, 148–167 (2016).
Gleason, H. A. The individualistic concept of the plant association. Bull. Torrey Bot. Soc. 53, 7–26 (1926).
Zimmer, A. et al. Time lag between glacial retreat and upward migration alters tropical alpine communities. Perspect. Plant Ecol. Evol. Syst. 30, 89–102 (2018).
Bayle, A. et al. Local environmental context drives heterogeneity of early succession dynamics in alpine glacier forefields. Biogeosciences 20, 1649–1669 (2023).
Li, S. et al. Convergence and divergence in a long-term old-field succession: the importance of spatial scale and species abundance. Ecol. Lett. 19, 1101–1109 (2016).
Fukami, T., Martijn Bezemer, T., Mortimer, S. R. & Putten, W. H. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 8, 1283–1290 (2005).
Marteinsdóttir, B., Thórhallsdóttir, T. E. & Svavarsdóttir, K. An experimental test of the relationship between small-scale topography and seedling establishment in primary succession. Plant Ecol. 214, 1007–1015 (2013).
Matthews, J. A., Hill, J. L., Winkler, S., Owen, G. & Vater, A. E. Autosuccession in alpine vegetation: testing the concept on an altitudinal bioclimatic gradient, Jotunheimen, southern Norway. CATENA 170, 169–182 (2018).
Ficetola, G. F. et al. Dynamics of ecological communities following current retreat of glaciers. Annu. Rev. Ecol. Evol. Syst. 52, 405–426 (2021).
Svoboda, J. & Henry, G. H. R. Succession in marginal Arctic environments. Arct. Alp. Res. 19, 373–384 (1987).
Zemp, M. et al. Global glacier mass changes and their contributions to sea-level rise from 1961 to 2016. Nature 568, 382–386 (2019).
Bosson, J. B. et al. Future emergence of new ecosystems caused by glacial retreat. Nature 620, 562–569 (2023).
Wojcik, R., Eichel, J., Bradley, J. A. & Benning, L. G. How allogenic factors affect succession in glacier forefields. Earth Sci. Rev. 218, 103642 (2021).
Fischer, A., Fickert, T., Schwaizer, G., Patzelt, G. & Groß, G. Vegetation dynamics in Alpine glacier forelands tackled from space. Sci. Rep. 9, 13918 (2019).
Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems (Springer, 2021).
Rosero, P. et al. Multi‐taxa colonisation along the foreland of a vanishing equatorial glacier. Ecography 44, 1010–1021 (2021).
Llambí, L. D. et al. Vegetation assembly, adaptive strategies and positive interactions during primary succession in the forefield of the last Venezuelan glacier. Front. Ecol. Evol. 9, 657755 (2021).
Hanusch, M., He, X., Ruiz-Hernández, V. & Junker, R. R. Succession comprises a sequence of threshold-induced community assembly processes towards multidiversity. Commun. Biol. 5, 424 (2022).
Erschbamer, B., Niederfriniger Schlag, R. & Winkler, E. Colonization processes on a central Alpine glacier foreland. J. Veg. Sci. 19, 855–862 (2008).
Losapio, G. et al. The consequences of glacier retreat are uneven between plant species. Front. Ecol. Evol. 8, 616562 (2021).
Koffel, T., Boudsocq, S., Loeuille, N. & Daufresne, T. Facilitation- vs. competition-driven succession: the key role of resource-ratio. Ecol. Lett. 21, 1010–1021 (2018).
Callaway, R. M. et al. Positive interactions among alpine plants increase with stress. Nature 417, 844–848 (2002).
Bertness, M. D. & Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 9, 191–193 (1994).
Walker, L. R., Clarkson, B. D., Silvester, W. B. & Clarkson, B. R. Colonization dynamics and facilitative impacts of a nitrogen-fixing shrub in primary succession. J. Veg. Sci. 14, 277–290 (2003).
Chapin, F. S., Walker, L. R., Fastie, C. L. & Sharman, L. C. Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecol. Monogr. 64, 149–175 (1994).
Gerla, D. J., Mooij, W. M. & Huisman, J. Photoinhibition and the assembly of light-limited phytoplankton communities. Oikos 120, 359–368 (2011).
Losapio, G. et al. Network motifs involving both competition and facilitation predict biodiversity in alpine plant communities. Proc. Natl Acad. Sci. USA 118, e2005759118 (2021).
Erschbamer, B. & Caccianiga, M. S. In Progress in Botany Vol. 78 (eds Cánovas, F. M. et al.) 259–284 (Springer, 2016).
Grime, J. P. Plant Strategies, Vegetation Processes, and Ecosystem Properties (Wiley, 2001).
Pérez, C. A. et al. Ecosystem development in short-term postglacial chronosequences: N and P limitation in glacier forelands from Santa Inés Island, Magellan Strait. Austral Ecol. 39, 288–303 (2014).
Pothula, S. K. & Adams, B. J. Community assembly in the wake of glacial retreat: a meta-analysis. Glob. Change Biol. 28, 6973–6991 (2022).
Anthelme, F., Carrasquer, I., Ceballos, J. L. & Peyre, G. Novel plant communities after glacial retreat in Colombia: (many) losses and (few) gains. Alp. Bot. 132, 211–222 (2022).
Whittaker, R. H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 30, 279–338 (1960).
Johnson, E. A. & Miyanishi, K. Testing the assumptions of chronosequences in succession. Ecol. Lett. 11, 419–431 (2008).
Ariza, M. et al. Plant biodiversity assessment through soil eDNA reflects temporal and local diversity. Methods Ecol. Evol. 14, 415–430 (2023).
Yoccoz, N. G. et al. DNA from soil mirrors plant taxonomic and growth form diversity. Mol. Ecol. 21, 3647–3655 (2012).
Foucher, A. et al. Persistence of environmental DNA in cultivated soils: implication of this memory effect for reconstructing the dynamics of land use and cover changes. Sci. Rep. 10, 10502 (2020).
Taberlet, P., Coissac, E., Hajibabaei, M. & Rieseberg, L. H. Environmental DNA. Mol. Ecol. 21, 1789–1793 (2012).
Zinger, L. et al. DNA metabarcoding—need for robust experimental designs to draw sound ecological conclusions. Mol. Ecol. 28, 1857–1862 (2019).
Johnson, M. D. et al. Environmental DNA as an emerging tool in botanical research. Am. J. Bot. 110, e16120 (2023).
Banerjee, P. et al. Environmental DNA analysis as an emerging non-destructive method for plant biodiversity monitoring: a review. AoB Plants 14, plac031 (2022).
Carvalho, J. C., Cardoso, P. & Gomes, P. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns: partitioning beta diversity. Glob. Ecol. Biogeogr. 21, 760–771 (2012).
Raffl, C., Mallaun, M., Mayer, R. & Erschbamer, B. Vegetation succession pattern and diversity changes in a glacier valley, Central Alps, Austria. Arct. Antarct. Alp. Res. 38, 421–428 (2006).
Tscherko, D., Hammesfahr, U., Zeltner, G., Kandeler, E. & Böcker, R. Plant succession and rhizosphere microbial communities in a recently deglaciated alpine terrain. Basic Appl. Ecol. 6, 367–383 (2005).
Kaufmann, R. Invertebrate succession on an alpine glacier foreland. Ecology 82, 2261–2278 (2001).
Cauvy-Fraunié, S. & Dangles, O. A global synthesis of biodiversity responses to glacier retreat. Nat. Ecol. Evol. 3, 1675–1685 (2019).
Zanzottera, M., Dalle Fratte, M., Caccianiga, M., Pierce, S. & Cerabolini, B. E. L. Community-level variation in plant functional traits and ecological strategies shapes habitat structure along succession gradients in alpine environment. Community Ecol. 21, 55–65 (2020).
Anthelme, F., Cauvy-Fraunié, S., Francou, B., Cáceres, B. & Dangles, O. Living at the edge: increasing stress for plants 2–13 years after the retreat of a tropical glacier. Front. Ecol. Evol. 9, 584872 (2021).
Erschbamer, B. & Mayer, R. Can successional species groups be discriminated based on their life history traits? A study from a glacier foreland in the Central Alps. Plant Ecol. Divers. 4, 341–351 (2011).
Gobbi, M. et al. Plant adaptive responses during primary succession are associated with functional adaptations in ground beetles on deglaciated terrain. Community Ecol. 11, 223–231 (2010).
Chase, J. M. & Myers, J. A. Disentangling the importance of ecological niches from stochastic processes across scales. Phil. Trans. R. Soc. B 366, 2351–2363 (2011).
del Moral, R. Increasing deterministic control of primary succession on Mount St. Helens, Washington. J. Veg. Sci. 20, 1145–1154 (2009).
Matthews, J. A. The Ecology of Recently Deglaciated Terrain. A Geoecological Approach to Glacier Forelands and Primary Succession (Cambridge Univ. Press, 1992).
Paterno, G. B., Siqueira Filho, J. A. & Ganade, G. Species-specific facilitation, ontogenetic shifts and consequences for plant community succession. J. Veg. Sci. 27, 606–615 (2016).
Brambilla, M. & Gobbi, M. A century of chasing the ice: delayed colonisation of ice-free sites by ground beetles along glacier forelands in the Alps. Ecography 37, 33–42 (2014).
Gobbi, M., Fontaneto, D. & De Bernardi, F. Influence of climate changes on animal communities in space and time: the case of spider assemblages along an alpine glacier foreland. Glob. Change Biol. 12, 1985–1992 (2006).
Delgado-Baquerizo, M. et al. Changes in belowground biodiversity during ecosystem development. Proc. Natl Acad. Sci. USA 116, 6891–6896 (2019).
Carrasco-Puga, G. et al. Revealing hidden plant diversity in arid environments. Ecography 44, 98–111 (2021).
Hartvig, I., Kosawang, C., Kjær, E. D. & Nielsen, L. R. Detecting rare terrestrial orchids and associated plant communities from soil samples with eDNA methods. Biodivers. Conserv. 30, 3879–3901 (2021).
Edwards, M. E. et al. Metabarcoding of modern soil DNA gives a highly local vegetation signal in Svalbard tundra. Holocene 28, 2006–2016 (2018).
Wang, H., Qi, J., Xiao, D., Wang, Z. & Tian, K. A re-evaluation of dilution for eliminating PCR inhibition in soil DNA samples. Soil Biol. Biochem. 106, 109–118 (2017).
Calderón-Sanou, I., Münkemüller, T., Boyer, F., Zinger, L. & Thuiller, W. From environmental DNA sequences to ecological conclusions: how strong is the influence of methodological choices? J. Biogeogr. 47, 193–206 (2019).
Walker, L. R., Wardle, D. A., Bardgett, R. D. & Clarkson, B. D. The use of chronosequences in studies of ecological succession and soil development: chronosequences, succession and soil development. J. Ecol. 98, 725–736 (2010).
Albrecht, M., Riesen, M. & Schmid, B. Plant-pollinator network assembly along the chronosequence of a glacier foreland. Oikos 119, 1610–1624 (2010).
Urban, M. C. et al. Improving the forecast for biodiversity under climate change. Science 353, aad8466 (2016).
Guerrieri, A. et al. Local climate modulates the development of soil nematode communities after glacier retreat. Glob. Chang. Biol. 30, e17057 (2024)
Marta, S. et al. The retreat of mountain glaciers since the little ice age: a spatially explicit database. Data 6, 107 (2021).
Guerrieri, A. et al. Effects of soil preservation for biodiversity monitoring using environmental DNA. Mol. Ecol. 30, 3313–3325 (2021).
Taberlet, P. et al. Soil sampling and isolation of extracellular DNA from large amount of starting material suitable for metabarcoding studies. Mol. Ecol. 21, 1816–1820 (2012).
Taberlet, P. et al. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Res. 35, e14 (2007).
Taberlet, P., Bonin, A., Zinger, L. & Coissac, E. Environmental DNA for Biodiversity Research and Monitoring (Oxford Univ. Press, 2018).
Ficetola, G. F. et al. Replication levels, false presences and the estimation of the presence/absence from eDNA metabarcoding data. Mol. Ecol. Resour. 15, 543–556 (2015).
Boyer, F. et al. OBITools: a unix-inspired software package for DNA metabarcoding. Mol. Ecol. Resour. 16, 176–182 (2016).
Bonin, A., Guerrieri, A. & Ficetola, G. F. Optimal sequence similarity thresholds for clustering of molecular operational taxonomic units in DNA metabarcoding studies. Mol. Ecol. Resour. 23, 368–381 (2023).
Ficetola, G. F. et al. An in silico approach for the evaluation of DNA barcodes. BMC Genomics 11, 434 (2010).
Bálint, M. et al. Millions of reads, thousands of taxa: microbial community structure and associations analyzed via marker genes. FEMS Microbiol. Rev. 40, 686–700 (2016).
Caccianiga, M., Luzzaro, A., Pierce, S., Ceriani, R. M. & Cerabolini, B. The functional basis of a primary succession resolved by CSR classification. Oikos 112, 10–20 (2006).
Dickie, I. A. et al. Towards robust and repeatable sampling methods in eDNA based studies. Mol. Ecol. Resour. 18, 940–952 (2018).
Baselga, A. & Leprieur, F. Comparing methods to separate components of beta diversity. Methods Ecol. Evol. 6, 1069–1079 (2015).
Baselga, A. Partitioning the turnover and nestedness components of beta diversity: partitioning beta diversity. Glob. Ecol. Biogeogr. 19, 134–143 (2010).
Legendre, P. & De Cáceres, M. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol. Lett. 16, 951–963 (2013).
Cardoso, P., Rigal, F. & Carvalho, J. C. BAT – Biodiversity Assessment Tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol. Evol. 6, 232–236 (2015).
Baselga, A. & Orme, C. D. L. betapart : an R package for the study of beta diversity: Betapart package. Methods Ecol. Evol. 3, 808–812 (2012).
Manly, B. F. J. Randomization, Bootstrap and Monte Carlo Methods in Biology (Chapman and Hall/CRC, 2017).
Smithson, M. & Verkuilen, J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 11, 54–71 (2006).
Bürkner, P.-C. brms: an R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017).
Muggeo, V. M. R. segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25 (2008).
Ficetola, G. F. & Denoël, M. Ecological thresholds: an assessment of methods to identify abrupt changes in species–habitat relationships. Ecography 32, 1075–1084 (2009).
Poorter, L. et al. Multidimensional tropical forest recovery. Science 374, 1370–1376 (2021).
Fickert, T. & Grüninger, F. High-speed colonization of bare ground – permanent plot studies on primary succession of plants in recently deglaciated glacier forelands. Land Degrad. Dev. 29, 2668–2680 (2018).
Guerrieri, A., Bonin, A., Gielly, L. & Ficetola, F. Raw sequencing data for studying the colonization of soil communities after glacier retreat (2022). https://doi.org/10.5281/zenodo.7628236
Acknowledgements
This study was funded by the European Research Council under the European Community’s Horizon 2020 Programme, Grant Agreement no. 772284 (IceCommunities) to I.C., A.C., A.G., S.M., A.B., R.A., R.S.A., F.G., L.G., N.K., G.A.D., J.P., W.T., M.C. and G.F.F. This research was also funded by Biodiversa+, the European Biodiversity Partnership under the 2021–2022 BiodivProtect joint call for research proposals, co-funded by the European Commission (grant agreement no. 101052342 ‘PrioritIce-Vanishing habitats: conservation priorities for glacier-related biodiversity threatened by climate change’) to I.C., R.A., W.T., M.C., M.G. and G.F.F. and with the funding organizations MUR and ANR. We thank R. Kaufmann, A. Guisan, K. Sieron and M. A. Morales-Martínez for help and discussions at various phases of this project.
Author information
Authors and Affiliations
Contributions
I.C., M.C. and G.F.F. conceived, developed and wrote the paper, with input from A.C., R. A., F.A., S.C.-F., M.G., A.R., A. Zerboni, P.T., J.P. and W.T.; I.C. performed the statistical analyses; A.G., S.M., A.B., F.G. and G.F.F. contributed to data preparation and curation; A.G., A.B. and L.G. performed laboratory analyses; A.G., S.M., A.B., R.A., F.A., R.S.A., P.A., P.A.G., S.C.-F., J.L.C.L, P.C., M.C.S., J.C., J.A.C.R., C.C., R.C.E., O.D., A.E., S.E., A.F., L.G., F.G., M.G, S.H., N.K., R.I.M., G.P., F.P., A.R., N.U., Y.Y., V.Z., A. Zerboni, A. Zimmer, G.A.D., J.P. M.C. and G.F.F. participated in sampling and the initial development of the study. All authors reviewed and/or provided input on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Ingolf Kühn, Robert Junker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Patterns of β-diversity and its components over time measured with temporal data to support the conclusions based on the chronosequence approach.
Data was obtained from Fickert & Grüninger94, which sampled vascular plants with traditional methods in permanent plots during the first decade after the deglaciation of two glaciers in the Alps (N = 30 comparisons). a. β-diversity components. Boxplots indicate median (middle line), 25th, 75th percentiles (box), as well as 1.5 * interquartile range (whiskers) and outliers (dots). Diamonds indicate the mean values. b. Results of the Bayesian generalized mixed models assessing the effects of mean age and age differences between compared sites on the different β-diversity measures. Glacier identity and identity of sites involved in the comparisons were included as random factors. Parameters with 95% CI non-overlapping zero are highlighted in bold.
Supplementary information
Supplementary Information
Supplementary Tables 1–8 and Figs. 1–11.
Supplementary Table 1
Supplementary Tables 1–3, with legends indicated in the Supplementary information file ‘Cantera_SI.pdf’.
Supplementary Code 1
Supplementary code.
Supplementary Code 2
Supplementary code.
Supplementary Code 3
Supplementary code.
Supplementary Code 4
Supplementary code.
Supplementary Code 5
Supplementary code.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cantera, I., Carteron, A., Guerrieri, A. et al. The importance of species addition ‘versus’ replacement varies over succession in plant communities after glacier retreat. Nat. Plants 10, 256–267 (2024). https://doi.org/10.1038/s41477-023-01609-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01609-4