Abstract
The staple food crop winter bread wheat (Triticum aestivum) acquires competence to flower in late spring after experiencing prolonged cold in temperate winter seasons, through the physiological process of vernalization. Prolonged cold exposure results in transcriptional repression of the floral repressor VERNALIZATION 2 (TaVRN2) and activates the expression of the potent floral promoter VERNALIZATION 1 (TaVRN1). Cold-induced TaVRN1 activation and TaVRN2 repression are maintained in post-cold vegetative growth and development, leading to an epigenetic ‘memory of winter cold’, enabling spring flowering. When and how the cold memory is reset in wheat is essentially unknown. Here we report that the cold-induced TaVRN1 activation is inherited by early embryos, but reset in subsequent embryo development, whereas TaVRN2 remains silenced through seed development, but is reactivated rapidly by light during seed germination. We further found that a chromatin reader mediates embryonic resetting of TaVRN1 and that chromatin modifications play an important role in the regulation of TaVRN1 expression and thus the floral transition, in response to developmental state and environmental cues. The findings define a two-step molecular mechanism for re-establishing vernalization requirement in common wheat, ensuring that each generation must experience winter cold to acquire competence to flower in spring.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings of this study are available within the paper and its supplementary information. Source data are provided with this paper.
References
Bouche, F., Woods, D. P. & Amasino, R. M. Winter memory throughout the plant kingdom: different paths to flowering. Plant Physiol. 173, 27–35 (2017).
Andres, F. & Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 13, 627–639 (2012).
Xu, S. & Chong, K. Remembering winter through vernalisation. Nat. Plants 4, 997–1009 (2018).
Michaels, S. D. & Amasino, R. M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11, 949–956 (1999).
Sheldon, C. C. et al. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11, 445–458 (1999).
Li, Z., Jiang, D. & He, Y. FRIGIDA establishes a local chromosomal environment for FLOWERING LOCUS C mRNA production. Nat. Plants 4, 836–846 (2018).
Choi, K. et al. The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell 23, 289–303 (2011).
Searle, I. et al. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 20, 898–912 (2006).
Angel, A., Song, J., Dean, C. & Howard, M. A Polycomb-based switch underlying quantitative epigenetic memory. Nature 476, 105–108 (2011).
Gao, Z. et al. A pair of readers of bivalent chromatin mediate formation of Polycomb-based 'memory of cold' in plants. Mol. Cell 83, 1109–1124 (2023).
Zhu, D. et al. Distinct chromatin signatures in the Arabidopsis male gametophyte. Nat. Genet. 55, 706–720 (2023).
Luo, X., Ou, Y., Li, R. J. & He, Y. H. Maternal transmission of the epigenetic ‘memory of winter cold’ in Arabidopsis. Nat. Plants 6, 1211–1218 (2020).
Borg, M. et al. Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat. Cell Biol. 22, 621–629 (2020).
Tao, Z. et al. Embryonic epigenetic reprogramming by a pioneer transcription factor in plants. Nature 551, 124–128 (2017).
Tao, Z. et al. Embryonic resetting of the parental vernalized state by two B3 domain transcription factors in Arabidopsis. Nat. Plants 5, 424–435 (2019).
McKeown, M., Schubert, M., Marcussen, T., Fjellheim, S. & Preston, J. C. Evidence for an early origin of vernalization responsiveness in temperate Pooideae grasses. Plant Physiol. 172, 416–426 (2016).
Distelfeld, A., Li, C. & Dubcovsky, J. Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 12, 178–184 (2009).
Trevaskis, B., Hemming, M. N., Dennis, E. S. & Peacock, W. J. The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci. 12, 352–357 (2007).
Trevaskis, B., Bagnall, D. J., Ellis, M. H., Peacock, W. J. & Dennis, E. S. MADS box genes control vernalization-induced flowering in cereals. Proc. Natl Acad. Sci. USA 100, 13099–13104 (2003).
Yan, L. et al. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl Acad. Sci. USA 100, 6263–6268 (2003).
Distelfeld, A., Tranquilli, G., Li, C., Yan, L. & Dubcovsky, J. Genetic and molecular characterization of the VRN2 loci in tetraploid wheat. Plant Physiol. 149, 245–257 (2009).
Yan, L. et al. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303, 1640–1644 (2004).
Yan, L. et al. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl Acad. Sci. USA 103, 19581–19586 (2006).
Corbesier, L. et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033 (2007).
Trevaskis, B. The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Funct. Plant Biol. 37, 479–487 (2010).
Kippes, N. et al. Identification of the VERNALIZATION 4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc. Natl Acad. Sci. USA 112, E5401–E5410 (2015).
Fu, D. et al. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genomics 273, 54–65 (2005).
Yan, L. et al. Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor. Appl. Genet. 109, 1677–1686 (2004).
Konopatskaia, I., Vavilova, V., Kondratenko, E. Y., Blinov, A. & Goncharov, N. P. VRN1 genes variability in tetraploid wheat species with a spring growth habit. BMC Plant Biol. 16, 244 (2016).
Xiao, J. et al. O-GlcNAc-mediated interaction between VER2 and TaGRP2 elicits TaVRN1 mRNA accumulation during vernalization in winter wheat. Nat. Commun. 5, 4572 (2014).
Fan, M. et al. O-linked N-acetylglucosamine transferase is involved in fine regulation of flowering time in winter wheat. Nat. Commun. 12, 2303 (2021).
Kippes, N. et al. Single nucleotide polymorphisms in a regulatory site of VRN-A1 first intron are associated with differences in vernalization requirement in winter wheat. Mol. Genet. Genomics 293, 1231–1243 (2018).
Xu, S. et al. The vernalization-induced long non-coding RNA VAS functions with the transcription factor TaRF2b to promote TaVRN1 expression for flowering in hexaploid wheat. Mol. Plant 14, 1525–1538 (2021).
Xie, L. et al. TaVrt2, an SVP-like gene, cooperates with TaVrn1 to regulate vernalization-induced flowering in wheat. New Phytol. 231, 834–848 (2021).
Dubcovsky, J. et al. Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Mol. Biol. 60, 469–480 (2006).
Chen, A. & Dubcovsky, J. Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet. 8, e1003134 (2012).
Shimada, S. et al. A genetic network of flowering-time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T. Plant J. 58, 668–681 (2009).
Deng, W. W. et al. Direct links between the vernalization response and other key traits of cereal crops. Nat. Commun. 6, 5882 (2015).
Tanaka, C. et al. Direct interaction between VRN1 protein and the promoter region of the wheat FT gene. Genes Genet. Syst. 93, 25–29 (2018).
Dixon, L. E. et al. VERNALIZATION1 controls developmental responses of winter wheat under high ambient temperatures. Development 146, dev172684 (2019).
Diallo, A. O., Ali-Benali, M. A., Badawi, M., Houde, M. & Sarhan, F. Expression of vernalization responsive genes in wheat is associated with histone H3 trimethylation. Mol. Genet. Genomics 287, 575–590 (2012).
Oliver, S. N., Finnegan, E. J., Dennis, E. S., Peacock, W. J. & Trevaskis, B. Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION1 gene. Proc. Natl Acad. Sci. USA 106, 8386–8391 (2009).
Huan, Q., Mao, Z., Chong, K. & Zhang, J. Global analysis of H3K4me3/H3K27me3 in Brachypodium distachyon reveals VRN3 as critical epigenetic regulation point in vernalization and provides insights into epigenetic memory. New Phytol. 219, 1373–1387 (2018).
Woods, D. P. et al. Establishment of a vernalization requirement in Brachypodium distachyon requires REPRESSOR OF VERNALIZATION1. Proc. Natl Acad. Sci. USA 114, 6623–6628 (2017).
Oliver, S. N., Deng, W. W., Casao, M. C. & Trevaskis, B. Low temperatures induce rapid changes in chromatin state and transcript levels of the cereal VERNALIZATION1 gene. J. Exp. Bot. 64, 2413–2422 (2013).
Khan, A. R. et al. Vernalization treatment induces site-specific DNA hypermethylation at the VERNALIZATION-A1 (VRN-A1) locus in hexaploid winter wheat. BMC Plant Biol. 13, 209 (2013).
Shi, X. et al. Comparative genomic and transcriptomic analyses uncover the molecular basis of high nitrogen-use efficiency in the wheat cultivar Kenong 9204. Mol. Plant 15, 1440–1456 (2022).
Wang, D. et al. Boosting wheat functional genomics via an indexed EMS mutant library of KN9204. Plant Commun. 4, 100593 (2023).
Mylne, J. S. et al. LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc. Natl Acad. Sci. USA 103, 5012–5017 (2006).
Finnegan, E. J., Genger, R. K., Kovac, K., Peacock, W. J. & Dennis, E. S. DNA methylation and the promotion of flowering by vernalization. Proc. Natl Acad. Sci. USA 95, 5824–5829 (1998).
Schiessl, S. V., Quezada-Martinez, D., Tebartz, E., Snowdon, R. J. & Qian, L. W. The vernalisation regulator FLOWERING LOCUS C is differentially expressed in biennial and annual Brassica napus. Sci. Rep. 9, 14911 (2019).
Wang, F. et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 14, 22–29 (2012).
Nelms, B. & Walbot, V. Gametophyte genome activation occurs at pollen mitosis I in maize. Science 375, 424–429 (2022).
Aoki, N. et al. Pathway of sugar transport in germinating wheat seeds. Plant Physiol. 141, 1255–1263 (2006).
Ishida, Y., Tsunashima, M., Hiei, Y. & Komari, T. Wheat (Triticum aestivum L.) transformation using immature embryos. Methods Mol. Biol. 1223, 189–198 (2015).
Ling, H. Q. et al. Genome sequence of the progenitor of wheat A subgenome Triticum urartu. Nature 557, 424–428 (2018).
Consortium, I. W. G. S. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345, 1251788 (2014).
Sato, K. et al. Chromosome-scale genome assembly of the transformation-amenable common wheat cultivar ‘Fielder’. DNA Res. 28, dsab008 (2021).
Yang, H. et al. Distinct phases of Polycomb silencing to hold epigenetic memory of cold in Arabidopsis. Science 357, 1142–1145 (2017).
Zhang, Y. Z. et al. Coupling of H3K27me3 recognition with transcriptional repression through the BAH–PHD–CPL2 complex in Arabidopsis. Nat. Commun. 11, 6212 (2020).
Li, J., Wang, Z., He, G., Ma, L. & Deng, X. W. CRISPR/Cas9-mediated disruption of TaNP1 genes results in complete male sterility in bread wheat. J. Genet. Genomics 47, 263–272 (2020).
Schuettengruber, B., Chourrout, D., Vervoort, M., Leblanc, B. & Cavalli, G. Genome regulation by Polycomb and trithorax proteins. Cell 128, 735–745 (2007).
Chen, K. et al. H3K36 methyltransferase SDG708 enhances drought tolerance by promoting abscisic acid biosynthesis in rice. New Phytol. 230, 1967–1984 (2021).
Jiang, P. et al. The COMPASS-like complex promotes flowering and panicle branching in rice. Plant Physiol. 176, 2761–2771 (2018).
Shilatifard, A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem. 81, 65–95 (2012).
McDaniel, S. L. & Strahl, B. D. Shaping the cellular landscape with Set2/SETD2 methylation. Cell. Mol. Life Sci. 74, 3317–3334 (2017).
Mozgova, I. & Hennig, L. The Polycomb group protein regulatory network. Annu. Rev. Plant Biol. 66, 269–296 (2015).
Yoo, S. D., Cho, Y. H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007).
Liu, Q. et al. Hi-TOM: a platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci. China Life Sci. 62, 1–7 (2019).
Wang, Y., Gu, X., Yuan, W., Schmitz, R. J. & He, Y. Photoperiodic control of the floral transition through a distinct Polycomb repressive complex. Dev. Cell 28, 727–736 (2014).
Louwers, M., Splinter, E., van Driel, R., de Laat, W. & Stam, M. Studying physical chromatin interactions in plants using Chromosome Conformation Capture (3C). Nat. Protoc. 4, 1216–1229 (2009).
Acknowledgements
We thank H. Lin (IGDB, Chinese Academy of Sciences) for kindly providing the seeds of KN9204 and T. urartu and J. Li and the in-house plant transformation facility for wheat transformation assistance. We thank G. Xu and X. You for experimental assistance. This work is supported in part by the Key R&D Program of Shandong Province, China (ZR202211070163 to Y.H.), the National Natural Science Foundation of China (grant numbers 31830049 and 31721001 to Y.H.) and the Peking-Tsinghua Center for Life Sciences.
Author information
Authors and Affiliations
Contributions
Y.H. conceived and supervised the research. D.N., Y.H. and Z.G. designed the experiments. D.N., Z.G. and Y.Z. performed the experiments. B.C. conducted bioinformatic analysis. D.N., Z.G. and Y.H. analysed the data. Y.H. wrote the paper with help from D.N.
Corresponding author
Ethics declarations
Competing interests
Y.H. and D.N. filed a patent application claiming the use of TaRVR1 genes in wheat genetic improvements. Z.G., B.C. and Y.Z. declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Richard Amasino and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of TaVRN1 and TaVRN2 in KN9204.
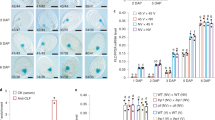
a, b, Analysis of vernalization response in the winter bread wheat cultivar KN9204. NV, non-vernalized; V6W, exposed to 6-week cold (4 °C). Plants were grown in LDs and at about 24 °C following cold exposure. ‘Days to flowering’ (with visible open florets) were scored in (b). Data are presented as mean values ± s.d. (n = 16). c, d, Percentage of each homeoallele of TaVRN1 in the leaves of seedlings cold-treated for 6 W (c) and TaVRN2 in the leaves of non-vernalized KN9204 seedlings (d). Data are presented as mean values ± s.d. of three biological replicates. n denotes the total number of transcripts sequenced in all three biological replicates. e, Schematic drawings of TaVRN2a and TaVRN2b. KN9204 contains two TaVRN2 loci, resulted from a duplication. Arrows indicate transcription start site (TSS), and exons are represented by black boxes. f, TaVRN2 protein sequence alignment. DNA-binding CCT domains (CONSTANS, CO-like, and TOC1) are underlined.
Extended Data Fig. 2 TaVRN1 and TaVRN2 expression over the course of vernalization in winter bread wheat.
a, TaVRN1, TaVRN2 and TaVRN3 expression in the leaves of cold-treated KN9204 seedlings. The first leaves of NV and cold-treated seedlings, the second leaves of V6W + 2 W (2w post cold), and the last leaves (flag leaves formed post cold) were harvested at ZT5 (5 hour /hr after light on). One-leaf seedlings were exposed to cold (in SDs) and returned to warmth for post-cold growth and development (in LDs). Transcripts were quantified by RT-qPCR and normalized directly to TaACT. b, c, Both TaVRN2 (b) and TaVRN3 (c) are specifically expressed in the leaf veins of KN9204 plants. Mesophyll cells and veins were isolated through enzymolysis. d, SAM (shoot apical meristem) of a 7-d-old KN9204 seedling prior to cold exposure. e, SAM of a KN9204 seedling exposed to cold for 6w. f, IM (inflorescence meristem) of a KN9204 seedling grown in LDs for 2w after cold. LS, lateral spikelet primordium. g, TaVRN1 and TaVRN2 expression in the SAM and IM tissues. Apexes were dissected under a microscope and tissues were harvested at ZT5. a-c, g, Data are presented as mean values ± s.d. of three biological replicates; u.d. for undetectable. P values in (a) are determined by one-way ANOVA with square root transformation of the data.
Extended Data Fig. 3 Analysis of TaVRN1 transcription in mature pollen grains and stability of TaVRN1 transcripts.
a, Micrograph of a mature pollen grain. Over twenty pollen grains were examined, and a representative micrograph is shown. VN, vegetative nucleus; SN, sperm nuclei. b, TaVRN1 expression in stamens (without pollen grains) and mature pollen gains. Transcripts were quantified by RT-qPCR and normalized directly to TaACT. Data are presented as mean values ± s.d. of three biological replicates. c, Ct (Cycle threshold) values of qPCR. Levels of TaVRN1 and three reference genes including TaACT, TaTUB2 (TUBULIN2) and TaPP2A (PROTEIN PHOSPHATASE SUBUNIT 2A) were quantified in stamen tissues and mature pollen grains. The expression levels of the three reference genes in the haploid pollen grains were significantly lower compared to the stamen tissues. Data are presented as mean values ± s.d. of three biological replicates d, Level of the unspliced nascent transcripts of TaVRN1 is relatively high in mature pollen grains. A promoter region of TaVRN1 serves as a control of genomic DNA. Three biological replicates (Rep) were examined. e-f, Stability analysis of spliced (e) and unspliced (f) TaVRN1 transcripts in late-stage stamens. Cordycepin was used to inhibit transcription. Transcripts were quantified by qPCR and directly normalized to TaACT. Data are presented as mean values ± s.d. of three biological replicates. P values are calculated using two-way ANOVA.
Extended Data Fig. 4 Developmental stages for embryo and grain in the hexaploid winter wheat KN9204.
Seedlings cold-treated for 6w were returned to normal growth temperature (about 24 °C) for post-cold growth and development. Grains at different stages were examined and photographed using a dissecting microscope (Leica S-APO) with a micro camera, while embryos were examined using a scanning electron microscope. D for days post anthesis (DPA). Note that wheat embryo development consists of pre-embryo, transition, leaf-early, leaf-mid, leaf-late and mature stages. Over eight samples at each stage were examined, and representative micrographs are shown. Co, coleoptile; Cr, coleorhiza; Epi, epiblast; Lr, lateral root sheath; SAM, shoot apical meristem; Sl, scutellum; Sus, suspensor.
Extended Data Fig. 5 Characterization of TaVRN2 expression during reproduction, seed germination and in the seedlings grown under different day lengths.
a, TaVRN2 expression is fully silenced during reproduction following vernalization. Data are presented as mean values ± s.d. of three biological replicates; u.d. for undetectable. b, Seed germination and seedling establishment under continuous dark (DD), short days (SD), long days (LD) and continuous light (LL). Seeds from vernalized KN9204 plants were imbibed for 2d. Inlets indicate the first leaves emerged out of a coleoptile. Scale bars (in seed germination images), 1 cm. c, d, Diurnal and rhythmic expression patterns of TaVRN2 in the first leaves of 5-d-old seedlings grown in LDs (c) and SDs (d). Transcripts were quantified by RT-qPCR and directly normalized to TaACT. Values are means ± s.d. of three biological replicates.
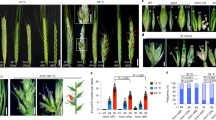
Extended Data Fig. 6 TaVRN2 expression is reactivated in the sprouting of rescued winter wheat embryos.
a, Immature embryos and the sprouting of rescued embryos. Immature embryos from vernalized KN9204 were rescued on half-strength MS media under LDs. On top are immature embryos, and at bottom are sprouted embryos upon rescue. Scale bars, 1 cm. b, Analysis of embryo sprouting in the course of immature embryo rescue. c, d, Analysis of TaVRN2 re-activation during sprouting of rescued immature embryos (10-d-old immature embryos on the rescue media for 14 d). Embryo sprouting stages are shown in (c). Total RNAs were extracted from shoots, the sections above the white lines shown in (c), and TaVRN2 transcripts were quantified and normalized to TaACT. Data are presented as mean values ± s.d. of three biological replicates; u.d. for undetectable. e, Analysis of TaVRN2 expression in embryo sprouting and young leaves. TaVRN2 transcripts extracted from immature embryos, shoots of sprouted rescued embryos and the third leaves of trilobate seedlings from rescued embryos, were quantified by RT-qPCR and normalized to TaACT. CK is the seedlings from normally-matured KN9204 seeds. Data are presented as mean values ± s.d. of three biological replicates; u.d. for undetectable. P values are calculated using one-way ANOVA. f, Flowering time of KN9204 plants from the rescued immature embryo. Plants were not exposed to cold. Values are means ± s.d. (n = 20). P values are calculated using one-way ANOVA with log transformation of the data.
Extended Data Fig. 7 Characterization of TaVRN1 and TaVRN2 expression in spring bread wheat.
a, b, Analysis of TaVRN1 (a) and TaVRN2 (b) expression in the life cycle of the spring wheat cultivar Fielder. Transcripts from indicated samples were quantified by qPCR and normalized to TaACT. c, Light-mediated TaVRN2 reactivation in early Fielder seedlings. Seeds were germinated under dark, and subsequently 5-d-old dark-grown seedlings were exposed to white light for up to 2 h, followed by quantification of TaVRN2 transcripts. a-c, Data are presented as mean values ± s.d. of three biological replicates; u.d. for undetectable.
Extended Data Fig. 8 Analysis of H3K4me3 and H3K27me3 on TaVRN1 and TaVRN2 chromatin.
a, ChIP-qPCR examination of H3K4me3 at TaVRN1 and the internal control TaAPSM1 (clathrin-associated adaptor protein complex 1 subunit mu-1). The levels of VRN1-In1 are as described in Fig. 5c. b, ChIP-qPCR examination of H3K27me3 at TaVRN1 and the internal control TaACT. The levels of VRN1-In1 in mature embryos and spikelets are as described as Fig. 5b. c, d, ChIP-qPCR examination of H3K27me3 (c) and H3K4me3 (d) at TaVRN2a. Levels of the immunoprecipitated TaVRN-A/B/D2a fragments by anti-H3K4me3 or anti-H3K27me3 were quantified by qPCR and directly normalized to input DNA. a-d, Data are presented as mean values ± s.d. of three biological replicates. P values in (b) are calculated using one-way ANOVA to compare multiple means (transformed by square root) or a two-tailed t-test for comparing two means. P values in (c-d) are calculated using one-way ANOVA.
Extended Data Fig. 9 Characterization of TaRVR1 and Tarvr1 mutants in winter bread wheat.
a, Schematic drawings of TaRVR-A1/B1/D1 in KN9204. Exons are indicated by black boxes, and arrows for transcription start site. Two red bars indicate the positions of single-guide RNAs in CRISRP/Cas9-mediated deletion. b, Schematic drawings of TaRVR-A1/B1/D1 proteins. Blue dotted boxes outline the BAH (bromo adjacent homology) and TFS2M (transcription elongation factor S-II) domains. c, Characterization of three independent Tarvr1-aabbdd mutants (Cas9 free). Two red lines indicate sgRNA target regions. Mutated amino acids (aa) were indicated by red; - for aa deletion and * for a premature stop codon. d, TaVRN1 expression in the seedlings exposed to cold for 4w. Total TaVRN1 transcripts were quantified by RT-qPCR and normalized to TaACT. e, TaRVR1 expression in the indicated samples. Levels of TaRVR1 transcripts were normalized to TaACT. f, TaVRN2 expression in the seedlings of WT and rvr1 mutants. NV, non-vernalized; V2W, cold-treated for 2w. d-f, Data are presented as mean values ± s.d. of three biological replicates. One-way ANOVA was conducted in (f); ns for not significant.
Extended Data Fig. 10 Analysis of the TaRVR-A1/B1/D1 proteins and chromatin-based regulation of TaVRN1 expression.
a, Domain architectures of AtAIPP3, BdRVR1 and TaRVR-A1/B1/D1 (from KN9204). b, Protein sequence alignment of AtAIPP3 and TaRVR-A1/B1/D1. Black- and grey-shaded residues indicate identical and similar residues, respectively. BAH domains are underlined with a blue line. Red stars denote the conserved key residues for H3K27me3 recognition. c, Structure superposition of BAH domains of AtAIPP3 (magenta) and TaRVR-A1/B1/D1 (cyan) using the Pymol program (https://pymol.org/2/). The BAH domain structure of TaRVR1 is modeled using the Phyre2 program (http://www.sbg.bio.ic.ac.uk/phyre2/). d, A working model for switching TaVRN1 chromatin state in response to prolonged cold and development state (embryogenesis) in the life cycle of winter bread wheat. Prior to cold, PRC2 deposits the repressive H3K27me3 marks on TaVRN1 chromatin. TaRVR1, likely in a complex with TaAIPP2 and TaCPL2 (Pol II dephosphorylation), functions together with Polycomb group factors to read and maintain the Polycomb-repressed state at TaVRN1, and thus to repress its repression. This, together with TaVRN2 expression in vascular bundles, confers winter-annual growth habit in wheat. Prolonged cold exposure in winter led to a strong reduction in H3K27me3 at TaVRN1 and consequent disruption of TaRVR1 function, and TaVRN1 chromatin is highly enriched with both H3K4me3 and H3K36me3. Hence, prolonged cold leads to a switching of the Polycomb-repressed state to a highly active state. Moreover, cold exposure may induce TaVRN1 expression through cold-responsive transcriptional regulators (TFs). Conceivably, these TFs may function in concert with TaRVR1 and chromatin modifiers to mediate cold induction of TaVRN1 expression. Upon embryonic resetting of TaVRN1 expression, TaVRN1 chromatin is switched back to Polycomb-repressed state. Note that the conserved COMPASS-like H3K4 methyltransferase complex and the H3K36 methyltransferase EFS are expected to deposit H3K4me3 and H3K36me3 at TaVRN1, respectively. S5(P) for Pol II phosphorylated on Serine 5.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1.
Source data
Source Data Figs. 2, 4 and 6 and Source Data Extended Data Figs. 2, 3, 6 and 8
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, D., Gao, Z., Cui, B. et al. A molecular mechanism for embryonic resetting of winter memory and restoration of winter annual growth habit in wheat. Nat. Plants 10, 37–52 (2024). https://doi.org/10.1038/s41477-023-01596-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01596-6