Abstract
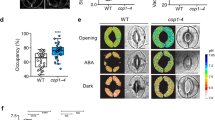
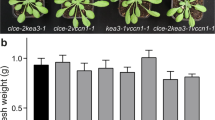
Stomata of most plants close to preserve water when the demand for CO2 by photosynthesis is reduced. Stomatal responses are slow compared with photosynthesis, and this kinetic difference erodes assimilation and water-use efficiency under fluctuating light. Despite a deep knowledge of guard cells that regulate the stoma, efforts to enhance stomatal kinetics are limited by our understanding of its control by foliar CO2. Guided by mechanistic modelling that incorporates foliar CO2 diffusion and mesophyll photosynthesis, here we uncover a central role for endomembrane Ca2+ stores in guard cell responsiveness to fluctuating light and CO2. Modelling predicted and experiments demonstrated a delay in Ca2+ cycling that was enhanced by endomembrane Ca2+-ATPase mutants, altering stomatal conductance and reducing assimilation and water-use efficiency. Our findings illustrate the power of modelling to bridge the gap from the guard cell to whole-plant photosynthesis, and they demonstrate an unforeseen latency, or ‘carbon memory’, of guard cells that affects stomatal dynamics, photosynthesis and water-use efficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated and analysed during this study are included in this published article and its supplementary information files and are also available on reasonable request to the corresponding author. Source data are provided with this paper.
Code availability
The OnGuard3 platform (v. 3.3.1.6) and the model parameter sets for wild-type and aca mutant Arabidopsis as binary code described herein are freely available and may be downloaded from www.psrg.org.uk.
References
Jezek, M. & Blatt, M. R. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol. 174, 487–519 (2017).
Lawson, T. & Blatt, M. R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570 (2014).
Roelfsema, M. R. & Hedrich, R. Making sense out of Ca2+ signals: their role in regulating stomatal movements. Plant Cell Environ. 33, 305–321 (2010).
Willmer, C. M. & Fricker, M. Stomata Vol. 2 (Chapman and Hall, 1996).
Buckley, T. N. & Mott, K. A. Dynamics of stomatal water relations during the humidity response: implications of two hypothetical mechanisms. Plant Cell Environ. 25, 407–419 (2002).
Shope, J. C., Peak, D. & Mott, K. A. Stomatal responses to humidity in isolated epidermes. Plant Cell Environ. 31, 1290–1298 (2008).
Ball, J. T., Woodrow, I. E. & Berry, J. A. in Progress in Photosynthesis Research Vol. 1 (ed. Biggens, J.) 221–224 (Martinus-Nijhoff, 1987).
Mott, K. A., Sibbernsen, E. D. & Shope, J. C. The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ. 31, 1299–1306 (2008).
Papanatsiou, M. et al. Optogenetic manipulation of stomatal kinetics improves carbon assimilation and water use efficiency. Science 363, 1456–1459 (2019).
Blatt, M. R. & Armstrong, F. K+ channels of stomatal guard cells: abscisic acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta 191, 330–341 (1993).
Irving, H. R., Gehring, C. A. & Parish, R. W. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc. Natl Acad. Sci. USA 89, 1790–1794 (1992).
Webb, A. A. R., McAinsh, M. R., Mansfield, T. A. & Hetherington, A. M. Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J. 9, 297–304 (1996).
Assmann, S. M. & Jegla, T. Guard cell sensory systems: recent insights on stomatal responses to light, abscisic acid, and CO2. Curr. Opin. Plant Biol. 33, 157–167 (2016).
Zhang, J. et al. Insights into the molecular mechanisms of CO2-mediated regulation of stomatal movements. Curr. Biol. 28, R1356–R1363 (2018).
Brearley, J., Venis, M. A. & Blatt, M. R. The effect of elevated CO2 concentrations on K+ and anion channels of Vicia faba L. guard cells. Planta 203, 145–154 (1997).
Wang, Y. et al. Unexpected connections between humidity and ion transport discovered using a model to bridge guard cell-to-leaf scales. Plant Cell 29, 2921–2139 (2017).
Pilot, G. et al. Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 276, 3215–3221 (2001).
Geiger, D. et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl Acad. Sci. USA 107, 8023–8028 (2010).
Brandt, B. et al. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl Acad. Sci. USA 109, 10593–10598 (2012).
Wang, C. et al. Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as CARBONIC ANHYDRASE4 interactor. Plant Cell 28, 568–582 (2016).
Hills, A., Chen, Z. H., Amtmann, A., Blatt, M. R. & Lew, V. L. OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol. 159, 1026–1042 (2012).
Chen, Z. H. et al. Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol. 159, 1235–1251 (2012).
Wang, Y. et al. Systems dynamic modelling of a guard cell Cl− channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol. 160, 1956–1972 (2012).
Jezek, M., Hills, A., Blatt, M. R. & Lew, V. L. A constraint–relaxation–recovery mechanism for stomatal dynamics. Plant Cell Environ. 42, 2399–2410 (2019).
Peak, D. & Mott, K. A. A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ. 34, 162–178 (2011).
Berry, J. A., Beerling, D. J. & Franks, P. J. Stomata: key players in the Earth system, past and present. Curr. Opin. Plant Biol. 13, 233–240 (2010).
Lawson, T., Simkin, A. J., Kelly, G. & Granot, D. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 203, 1064–1081 (2014).
Buckley, T. N. & Mott, K. A. Modelling stomatal conductance in response to environmental factors. Plant Cell Environ. 36, 1691–1699 (2013).
Vialet-Chabrand, S. et al. Global sensitivity analysis of OnGuard models identifies key hubs for transport interaction in stomatal dynamics. Plant Physiol. 174, 680–688 (2017).
Roelfsema, M. R. G. & Hedrich, R. In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol. 167, 665–691 (2005).
Kollist, H., Nuhkat, M. & Roelfsema, M. R. G. Closing gaps: linking elements that control stomatal movement. New Phytol. 203, 44–62 (2014).
Hu, H. H. et al. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 12, 87–90 (2010).
Morison, J. I. L. & Jarvis, P. G. Direct and indirect effects of light on stomata II. In Commelina communis L. Plant Cell Environ. 6, 103–109 (1983).
Zhu, J., Talbott, L. D., Jin, X. & Zeiger, E. The stomatal response to CO2 is linked to changes in guard cell zeaxanthin. Plant Cell Environ. 21, 813–820 (1998).
Hiyama, A. et al. Blue light and CO2 signals converge to regulate light-induced stomatal opening. Nat. Commun. https://doi.org/10.1038/s41467-017-01237-5 (2017).
Medeiros, D. B. et al. Enhanced photosynthesis and growth in atquac1 knockout mutants are due to altered organic acid accumulation and an increase in both stomatal and mesophyll conductance. Plant Physiol. 170, 86–101 (2016).
Merilo, E. et al. PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol. 162, 1652–1668 (2013).
Tian, W. et al. A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. https://doi.org/10.1038/ncomms7057 (2015).
Grabov, A. & Blatt, M. R. A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 119, 277–287 (1999).
Minguet-Parramona, C. et al. An optimal frequency in Ca2+ oscillations for stomatal closure Is an emergent property of ion transport in guard cells. Plant Physiol. 170, 32–45 (2016).
Grabov, A. & Blatt, M. R. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc. Natl Acad. Sci. USA 95, 4778–4783 (1998).
Blatt, M. R. Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 16, 221–241 (2000).
Boursiac, Y. et al. Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol. 154, 1158–1171 (2010).
Hwang, I., Sze, H. & Harper, J. F. A calcium-dependent protein kinase can inhibit a calmodulin- timulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc. Natl Acad. Sci. USA 97, 6224–6229 (2000).
Conn, S. J. et al. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23, 240–257 (2011).
Rahmati-Ishka, M. et al. Arabidopsis Ca2+-ATPases 1, 2, and 7 in the endoplasmic reticulum contribute to growth and pollen fitness. Plant Physiol. 185, 1966–1985 (2021).
Takemiya, A. et al. Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat. Commun. https://doi.org/10.1038/ncomms3094 (2013).
Isner, J. C., Begum, A., Nuehse, T., Hetherington, A. M. & Maathuis, F. J. M. KIN7 kinase regulates the vacuolar TPK1 K+ channel during stomatal closure. Curr. Biol. 28, 466–472 (2018).
Ando, E. & Kinoshita, T. Red light-induced phosphorylation of plasma membrane H+-ATPase in stomatal guard cells. Plant Physiol. 178, 838–849 (2018).
Farquhar, G. D. & Sharkey, T. D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 33, 317–345 (1982).
Endy, D. & Brent, R. Modelling cellular behaviour. Nature 409, 391–395 (2001).
Xue, S. et al. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 30, 1645–1658 (2011).
Merlot, S. et al. Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 26, 3216–3226 (2007).
McAinsh, M. R., Webb, A. A. R., Taylor, J. E. & Hetherington, A. M. Stimulus-induced oscillations in guard cell cytosolic-free calcium. Plant Cell 7, 1207–1219 (1995).
Garcia-Mata, C. et al. Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc. Natl Acad. Sci. USA 100, 11116–11121 (2003).
Thor, K. & Peiter, E. Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol. 204, 873–881 (2014).
Resentini, F., Ruberti, C., Grenzi, M., Bonza, M. & Costa, A. The signatures of organellar calcium. Plant Physiol. https://doi.org/10.1093/plphys/kiab189 (2021).
Yang, H. M., Zhang, X. Y., Wang, G. X. & Zhang, J. H. Water channels are involved in stomatal oscillations encoded by parameter-specific cytosolic calcium oscillations. J. Integr. Plant Biol. 48, 790–799 (2006).
Kaiser, H. & Kappen, L. Stomatal oscillations at small apertures: indications for a fundamental insufficiency of stomatal feedback-control inherent in the stomatal turgor mechanism. J. Exp. Bot. 52, 1303–1313 (2001).
Cowan, I. R. Oscillations in stomatal conductance and plant functioning associated with stomatal conductance: observations and a model. Planta 106, 185–219 (1972).
Lang, A. R. G., Klepper, B. & Cumming, M. J. Leaf water balance during oscillation of stomatal aperture. Plant Physiol. 44, 826–832 (1969).
Eckstein, J., Beyschlag, W., Mott, K. A. & Ryel, R. J. Changes in photon flux can induce stomatal patchiness. Plant Cell Environ. 19, 1066–1074 (1996).
Vialet-Chabrand, S. R. M. et al. Temporal dynamics of stomatal behavior: modeling and implications for photosynthesis and water use. Plant Physiol. 174, 603–613 (2017).
Yang, X. et al. A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytol. 207, 491–504 (2015).
Manzoni, S. et al. Hydraulic limits on maximum plant transpiration and the emergence of the safety–efficiency trade-off. New Phytol. 198, 169–178 (2013).
Vico, G., Manzoni, S., Palmroth, S. & Katul, G. Effects of stomatal delays on the economics of leaf gas exchange under intermittent light regimes. New Phytol. 192, 640–652 (2011).
Buckley, T. N. & Schymanski, S. J. Stomatal optimisation in relation to atmospheric CO2. New Phytol. 201, 372–377 (2014).
Lin, Y.-S. et al. Optimal stomatal behaviour around the world. Nat. Clim. Change 5, 459–464 (2015).
Schymanski, S. J., Roderick, M. L., Sivapalan, M., Hutley, L. B. & Beringer, J. A canopy-scale test of the optimal water-use hypothesis. Plant Cell Environ. 31, 97–111 (2008).
Ball, M. C. & Farquhar, G. D. Photosynthetic and stomatal responses of 2 mangrove species, Aegiceras corniculatum and Avicennia marina, to long-term salinity and humidity conditions. Plant Physiol. 74, 1–6 (1984).
Pieruschka, R., Huber, G. & Berry, J. A. Control of transpiration by radiation. Proc. Natl Acad. Sci. USA 107, 13372–13377 (2010).
Damour, G., Simonneau, T., Cochard, H. & Urban, L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 33, 1419–1438 (2010).
Lavergne, A. et al. Observed and modelled historical trends in the water-use efficiency of plants and ecosystems. Glob. Change Biol. 25, 2242–2257 (2019).
Keenan, T. F. et al. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499, 324–327 (2013).
Rasband, W. S. & Bright, D. S. NIH Image: a public domain image-processing program for the Macintosh. Microbeam Anal. 4, 137–149 (1995).
Alonso, J. M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 (2003).
Kleinboelting, N., Huep, G., Kloetgen, A., Viehoever, P. & Weisshaar, B. GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res. 40, D1211–D1215 (2012).
McElver, J. et al. Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana. Genetics 159, 1751–1763 (2001).
Chen, Z. H., Hills, A., Lim, C. K. & Blatt, M. R. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J. 61, 816–825 (2010).
Blatt, M. R. Electrical characteristics of stomatal guard cells: the contribution of ATP-dependent, “electrogenic” transport revealed by current-voltage and difference-current-voltage analysis. J. Membr. Biol. 98, 257–274 (1987).
MacRobbie, E. A. C. & Lettau, J. Ion content and aperture in isolated guard cells of Commelina communis L. J. Membr. Biol. 53, 199–205 (1980).
Squire, G. R. & Mansfield, T. A. Simple method of isolating stomata on detached epidermis by low pH treatment: observations of importance of subsidiary cells. New Phytol. 71, 1033–1043 (1972).
Kurebayashi, N., Harkins, A. B. & Baylor, S. M. Use of fura red as an intracellular calcium indicator in frog skeletal-muscle fibers. Biophys. J. 64, 1934–1960 (1993).
von Caemmerer, S. Steady-state models of photosynthesis. Plant Cell Environ. 36, 1617–1630 (2013).
Ogren, E. & Evans, J. R. Photosynthetic light-response curves: I. The influence of CO2 partial-pressure and leaf inversion. Planta 189, 182–190 (1993).
Morison, J. I. L. et al. Lateral diffusion of CO2 in leaves is not sufficient to support photosynthesis. Plant Physiol. 139, 254–266 (2005).
Parkhurst, D. F., Wong, S. C., Farquhar, G. D. & Cowan, I. R. Gradients of intercellular CO2 levels across the leaf mesophyll. Plant Physiol. 86, 1032–1037 (1988).
Marquardt, D. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 11, 431–441 (1963).
Acknowledgements
This work was supported by BBSRC grants BB/L001276/1, BB/L019205/1, BB/M001601/1 and BB/N01832X/1 to M.R.B. and by National Science Foundation grant IOS 1656774 to J.F.H. Y.W. was supported by the National Science Foundation of China grant 31871537 and the Central Universities Fundamental Research Fund 2020XZZX002-21. F.A.L.S.-A. was supported by a Lord Kelvin and Adam Smith PhD studentship. We thank A. Ruiz-Pardo for help in plant maintenance.
Author information
Authors and Affiliations
Contributions
M.R.B., A.H. and V.L.L. conceived the work and developed the model platform; A.H. encoded the platform; M.R.B., M.J., B.H. and Y.H. resolved the models; M.J., J.S. and Y.H. carried out gas exchange and aperture measurements; F.A.L.S.-A., N.D. and M.R.B. carried out growth studies, biochemical and Ca2+ analyses; Y.W. and F.A.L.S.-A. carried out voltage clamp experiments and M.J., Y.W. and F.A.L.S.-A. analysed the results with M.R.B.; M.R.B. wrote the manuscript with M.J., A.H., F.A.L.S.-A. and V.L.L.; all authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Toshinori Kinoshita and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Appendices 1–3 (including Supplementary Tables 1 and 2) and Figs. 1–9.
Source data
Source Data Fig. 2
Compiled statistical source data and analysis.
Source Data Fig. 4
Compiled statistical source data and analysis.
Source Data Fig. 5
Compiled statistical source data and analysis.
Source Data Fig. 6
Compiled statistical source data and analysis.
Rights and permissions
About this article
Cite this article
Jezek, M., Silva-Alvim, F.A.L., Hills, A. et al. Guard cell endomembrane Ca2+-ATPases underpin a ‘carbon memory’ of photosynthetic assimilation that impacts on water-use efficiency. Nat. Plants 7, 1301–1313 (2021). https://doi.org/10.1038/s41477-021-00966-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00966-2
This article is cited by
-
Modeling the effect of grazing on carbon and water use efficiencies in grasslands on the Qinghai–Tibet Plateau
BMC Ecology and Evolution (2024)
-
Effects of elevated CO2 on hydraulic performance and carbon assimilation of Schefflera arboricola
Journal of Soils and Sediments (2023)
-
Engineering a K+ channel ‘sensory antenna’ enhances stomatal kinetics, water use efficiency and photosynthesis
Nature Plants (2022)
-
Exploiting a channel voltage ‘antenna’ for gains in water use efficiency and biomass
Nature Plants (2022)