Abstract
The mycobacterial repressor, DarR, a TetR family regulator (TFR), was the first transcription regulator shown to bind c-di-AMP. However, the molecular basis for this interaction and the mechanism involved in DNA binding by DarR remain unknown. Here we describe DarR-c-di-AMP and DarR-DNA structures and complementary biochemical assays. The DarR-c-di-AMP structure reveals a unique effector binding site for a TFR, located between DarR dimer subunits. Strikingly, we show this motif also binds cAMP. The location of the adenine nucleotide binding site between subunits suggests this interaction may facilitate dimerization and hence DNA binding. Indeed, biochemical assays show cAMP enhances DarR DNA binding. Finally, DarR-DNA structures reveal a distinct TFR DNA-binding mechanism involving two interacting dimers on the DNA. Thus, the combined data unveil a newly described second messenger binding motif and DNA binding mode for this important family of regulators.
Similar content being viewed by others
Introduction
Single-celled organisms such as bacteria are faced with constantly changing environmental conditions. Hence, they must elicit appropriate adaptive responses to survive. This is accomplished via signal transduction systems. The most common prokaryotic signal transduction mechanisms involve one-component regulatory systems that are comprised of a single polypeptide containing a sensory domain and a DNA-binding domain. Among the best studied of the single component systems are the TetR family of regulators (TFRs)1,2,3,4. TFRs are the third most frequently occurring transcription factor family in bacteria and regulate diverse aspects of bacterial physiology. TFRs bind and respond to a wide range of effectors, which commonly function as inducers1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34. Inducers are ligands that bind and inactivate repressors from interacting with target DNA operator sites. TFRs are perhaps best known for their roles in multidrug resistance (MDR), through the regulation of multidrug efflux pumps1,2,3,4,5,6,7,8,9,10. Recently, DarR, a 201 residue, TFR regulator of a putative MDR transporter, Ms5347, in Mycobacteria was discovered and represents the focus of this work11.
DarR was found to not only regulate transcription of Ms5347, but also genes encoding enzymes involved in fatty acid synthesis and the cold shock protein, CspA11. DarR also regulates transcription of its own gene11. Consistent with a critical regulatory role for DarR in mycobacterial physiology, darR knockdown resulted in growth defects while its overexpression was toxic11. Interestingly, DarR was discovered in a screen in Mycobacteria smegmatis (now Mycolicibacterium smegmatis) for cyclic-di-AMP (c-di-AMP) binding factors11. Cyclic nucleotides, c-di-GMP and c-di-AMP, have recently emerged as key prokaryotic second messengers. While several c-di-GMP receptors have been discovered and characterized12,13,14,15,16, comparatively fewer c-di-AMP binding proteins have been identified. c-di-AMP is synthesized by diadenylate cyclases via the condensation of two ATP molecules and is degraded by three families of phosphodiesterases (PDEs)17,18,19. C-di-AMP can also be removed from the cell via export by specific MDR transporters17. The bacterial processes regulated by c-di-AMP are diverse, ranging from the maintenance of osmotic pressure, DNA damage responses, control of central metabolism to cold shock and regulation of fatty acid biosynthesis17,18,19,20,21,22,23. More recent studies showed that c-di-AMP is essential for the survival of several Gram-positive bacteria17,18,19.
The sequence of the mycobacterial c-di-AMP binding protein, DarR, places it in the TFR family of regulators11. TFR members contain a highly conserved structural organization1,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44 composed of 9 to 11 α-helices that form two domains, an N-terminal DNA-binding domain and a C-terminal inducer binding/dimerization domain1,2,4,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44. The DNA-binding domain is the most conserved region amongst TFR proteins and is composed of helices 1–3, where helices 2–3 form a helix-turn-helix (HTH) motif. The TFR C-terminal inducer binding/dimerization domain is sequentially less conserved but generally consists of a triangle-like ligand binding region composed of helices 5, 6, and 7 with the last two helices of this domain typically combining with the corresponding helices in the dimer mate to create a four-helix bundle dimerization module1,2,4.
TFR DNA binding requires dimerization, as all TetR proteins characterized to date bind palindromic DNA, including DarR1,2,4,11,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44. The DNA operator sites bound by DarR were identified by electrophoretic mobility shift assays, chromatin immunoprecipitation and DNase I protection experiments11. These operators, within promoters for the darR, Ms5347 and cspA genes, all contain a palindromic sequence of ATACT(N)4AGTAT (where the N indicates less conserved bases)11. Further studies revealed that DarR functions as a repressor11. Though DarR was identified as a c-di-AMP binding protein, it remains unknown how it binds this second messenger. Also unknown is how DarR recognizes its cognate DNA. To address these questions, we solved crystal structures of DarR bound to target DNA, c-di-AMP and performed complementary biochemical analyses. Our studies reveal c-di-AMP binds a newly described TFR ligand binding site in DarR. Furthermore, we show that DarR binds cAMP using the same motif and that cAMP significantly stimulates DNA binding by DarR. The DarR-DNA structure reveals a unique mode of DNA binding for a TFR protein that involves binding of two interacting DarR dimers. Thus, these studies underscore that while TFR members are among the best characterized transcription regulators, much remains to be learned about the molecular mechanisms of ligand and DNA binding by these proteins.
Results
Structure determination of M. smegmatis and Rhodococcus sp. USK13 DarR
To elucidate the molecular mechanisms by which DarR binds c-di-AMP and DNA we sought to obtain structures. To enhance the likelihood of obtaining crystals, we generated expression constructs for the M. smegmatis DarR and two other DarR orthologs. Specifically, the DarR proteins from Mycolicibacterium baixiangningiae and Rhodococcus sp. USK13, which share 84% and 82% sequence identity with M. smegmatis DarR, were also expressed and purified for crystallization trials. The N-terminal HTH DNA-binding regions of these proteins are conserved indicating a shared DNA binding mode (Supplementary Fig. 1). However, to analyze operator binding by these DarR proteins, we employed fluorescence polarization (FP). These studies assessed binding of the proteins to a double stranded (ds) DNA site containing the DarR operator, 5´-TAGATACTCCGGAGTATCTA-3 (the double stranded (ds) DNA site is formed with the complementary strand)11. These experiments showed that all three DarR proteins bound the DNA site with essentially the same affinity, i.e. Kds of 11.4 ± 1.2 nM, 12.4 ± 0.7 nM and 10.1 ± 1 nM for Rhodococcus sp. USK13 DarR, M. baixiangningiae DarR and M. smegmatis DarR, respectively (Supplementary Fig. 2; Methods).
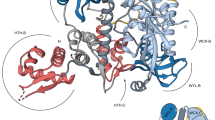
Crystals of M. smegmatis DarR and M. baixiangningiae DarR were produced that diffracted to 3.56 Å and 1.60 Å resolution, respectively. The M. baixiangningiae DarR structure was solved by selenomethionine single wavelength anomalous diffraction (SAD) and refined to final Rwork/Rfree values of 18.5%/20.2% to 1.6 Å. This structure was then used to determine the M. smegmatis DarR structure (Fig. 1a) (Supplementary Table 1). There are two M. smegmatis DarR dimers in the crystallographic asymmetric unit (ASU) and one DarR subunit in the M. baixiangningiae DarR structure. A M. baixiangningiae DarR dimer with the same assembly as the M. smegmatis DarR dimer is generated by crystallographic symmetry (Fig. 1a, b). Formation of the DarR dimer by interaction of the two monomers results in the burial of ~1300 Å2 of surface from solvent. The structures show that, as expected, DarR belongs to the TFR family of proteins, with DALI searches revealing the TetR member showing the strongest structural homology to DarR was the Thermus thermophilus HB8 PfmR protein (pdb code:3VPR). The DarR and PfmR subunits superimpose with a root mean square deviation (rmsd) of 1.9 Å for 160 corresponding Cα atoms (Supplementary Fig. 3). Similar to other TFRs, DarR has a two-domain architecture with an N-terminal, HTH containing domain and C-terminal inducer binding/dimerization domain1,2,4. The DarR N-terminal domain consists of helices 1-3 and the dimer domain is comprised of helices α4-α9 (Fig. 1a). In DarR, dimerization is mediated by helices α8 and α9, which interact with α8´ and α9´ (where the prime indicates the other subunit in the dimer) to form a four-helix bundle dimerization module.
a 1.6 Å resolution structure of the M. baixiangningiae DarR. One subunit is colored magenta and the other, green. Shown as sticks is the fortuitously bound ligand. Secondary structural elements are labeled for one subunit. b Top, superimposition of M. baixiangningiae DarR structure (green) onto the M. smegmatis structure (slate). Indicated below the structures is the distance between the recognition helices of each dimer (50 Å for the M. baixiangningiae DarR dimer and 41 Å for the M. smegmatis DarR dimer). Bottom, superimposition of the two M. smegmatis DarR dimers (one in slate and one in orange) that are present in the ASU showing they are essentially identical aside from loop regions. c Close-up of the fortuitously bound borate complex in the M. baixiangningiae DarR structure with Sigma-A weighted omit electron density (mFo-DFc) included (blue mesh) and contoured at 3.3 σ. The omit electron density was generated in Phenix by first removing the ligand and then subjecting the coordinates to 30 cycles of refinement to remove bias. The ligand and residues that bind the ligand are shown as sticks. d 2-D chemical structure of the Tris-borate-glycerol complex.
M. baixiangningiae DarR structure adopts inducer bound conformation
While the M. smegmatis and M. baixiangningiae DarR structures have essentially the same overall folds (Fig. 1b), the distances between their DNA-binding recognition helices differ (as measured by the distance between the two centrally located Tyr47 residues on each recognition helix). In M. baixiangningiae DarR the helices are separated by 50 Å while the distance between these helices of the M. smegmatis dimer is 41 Å (Fig. 1b). There are also conformational differences between the structures in residues 108-135. These residues are notably proximal to the ligand/inducer binding domains. However, both structures were obtained without added ligand. Analyses of the electron density in binding pockets in the M. smegmatis DarR structure showed water molecules. But there was no evident density for a ligand. By contrast, electron density with an unusual spirocyclic-like structure was found in the ligand-binding pocket of the M. baixiangningiae DarR structure near residues 108-138 (Fig. 1c; Supplementary Fig. 4). Searches revealed no metabolites from the E. coli expression system harboring such a structure. Based on the crystallization conditions and the chemistry of binding, the density was best fit to a complex of Tris buffer and glycerol coordinated by boron; Tris was present at a concentration of 100 mM in the crystallization solution, glycerol was in both the protein buffer and cryo-solvent and boron is a micronutrient in bacteria45 and other organisms and is also present in glassware. Such borate complexes were previously identified in solutions of Tris, glycerol and other polyhydroxy compounds and have been structurally characterized by NMR46.
The borate complex ligand possesses a partial negative charge (Fig. 1c, d), which is stabilized by an interaction with DarR residue Arg135 (Fig. 1c, d). In addition to Arg135, there are numerous DarR residues that contact the ligand, including Met66, His94, Asn97, His105, Val108, His109, Tyr138, Leu168 and Asn172 (Fig. 1c). Sequence alignments of DarR proteins reveals that most of these residues are conserved (Supplementary Fig. 5). The only ligand interacting residues not conserved among DarR homologs, Asn97 and Val108, have conservative substitutions that could make the same interactions. TFR proteins that regulate the transcription of MDR pump genes are known to bind substrates of their regulated pumps, which consist of a range of structurally dissimilar compounds1,2,4. DarR likely similarly binds a range of inducers with diverse structures that may resemble the borate complex. The substrates of the putative MDR pump regulated by DarR are currently unknown and hence future work will be needed to elucidate Ms5347 substrates and whether they function as DarR inducers.
DarR in complex with c-di-AMP
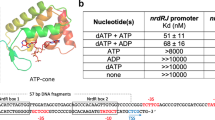
DarR was originally identified in a UV cross-linking assay as a c-di-AMP binding protein11. These studies showed that DarR specifically bound c-di-AMP, as it showed no binding to c-di-GMP11. C-di-AMP is generated in M. smegmatis by the DisA c-di-AMP cyclase (WP_011731023.1). We note that both Rhodococcus sp. USK13 and M. baixiangningiae possess similar DisA proteins, WP_109326508.1 and WP_197375332.1, respectively, which each share 93% sequence identity with the M. smegmatis DisA (Supplementary Fig. 6). To assess c-di-AMP binding to M. smegmatis and Rhodococcus sp. USK13 DarR proteins we utilized F-c-di-AMP (2′-O-(6-[Fluoresceinyl]aminohexylcarbamoyl)-cyclic diadenosine monophosphate) as a probe in FP studies. The proteins bound F-c-di-AMP with Kds of 21.6 ± 2.5 μM and 21.9 ± 2.7 μM, respectively (Fig. 2a; Supplementary Fig. 7). The Kds obtained with the fluoresceinated probe were higher (lower affinity) than the previously 2.3 μM reported by Zheng et al. 11, which is likely due to the attached fluorescein tag. However, the probe served as a useful reporter for subsequent experiments.
a Fluorescence polarization (FP) binding isotherms of WT Rhodococcus sp. USK13 DarR (red, open circles) and Rhodococcus sp. USK13 DarR(W177A-Q184A-L192A) (blue, open squares), respectively, to F-c-di-AMP. The x and y axes are concentration of Rhodococcus sp. USK13 DarR in μM and millipolarization units (mP), respectively. WT Rhodococcus sp. USK13 DarR bound with a Kd of 21.9 ± 2.7 μM, while the mutant showed no detectable binding. Data points represent mean values ± SD with the error bars centered at the mean. The error in overall Kd was determined as the SD between the calculated Kds for three runs. b Overall structure of the WT Rhodococcus sp. USK13 DarR-c-di-AMP complex. One subunit is colored magenta and the other green. Electron density (mFo-DFc) calculated after omitting the c-di-AMP (blue mesh) is included and contoured at 2.9 σ. Helices that contain residues contributing to c-di-AMP binding are labeled. c c-di-AMP mediated bridging between DarR dimers observed in the crystal packing. d and e Close-up of the two nucleotide binding interactions at each of the DarR dimer interfaces with the omit electron density included (blue mesh) and contoured at 2.9 σ. One DarR subunit is colored green and the other, magenta. Residues that participate in nucleotide binding are shown as sticks and labeled.
To elucidate the structural mechanism of c-di-AMP binding we obtained the Rhodococcus sp. USK13 DarR-c-di-AMP structure to 2.45 Å resolution (Supplementary Table 1). The structure revealed no density for c-di-AMP in the inducer binding pocket, but density for a di-adenine nucleotide was observed near the four-helix bundle module of DarR, with the nucleotide making contacts to both subunits of the dimer (Fig. 2b–e). The density for the adenine moieties was well resolved while the density for the phosphate groups was less clear (Fig. 2b–e; Supplementary Fig. 8). Hence, a di-adenine nucleotide was fitted to the map. The phosphate moieties of the c-di-AMP appear flexible, resulting in elevated B-factors (ave) of 99.0 Å2 for c-di-AMP compared to 72.2 Å2 for the protein and 71.6 Å2 for water molecules. In the structure, the dinucleotide is wedged within the long loop between helices α8 and α9 of one DarR subunit and also contacts residues from the N-terminus of α8´ from the other subunit (Fig. 2d–e).
In the DarR-c-di-AMP structure, a di-nucleotide is bound at each C-terminal end of the dimer (Fig. 2b, c). In this interaction, only one of the adenine bases is bound within each pocket and the other adenine base is solvent exposed (Fig. 2d–e). Each of the exposed adenine bases inserts into the binding pocket of an adjacent DarR dimer, leading to DarR polymer contacts in the crystal (Fig. 2c; Supplementary Fig. 9; Supplementary Fig. 10a, b). Comparisons of the two bound adenines in the dimer shows that the structure captured two interaction modes, in which each adenine is oriented slightly differently within the pocket. In one interaction, the adenine is specified by hydrogen bonds from the Gln184 Oε and Nε atoms to the adenine N6 and N7 atoms, respectively (Fig. 2d). In the other subunit, the N6 atom of the adenine contacts the Arg179 carbonyl oxygen and the Gln184 side chain Nε atom contacts the N7 atom (Fig. 2e). In addition to Gln184 and Arg179 both adenine moieties are contacted by the side chains of Trp177, Tyr178, Ile181 and Leu192 from one DarR subunit and Asp159 Leu162 and Arg165 from the other subunit (Supplementary Fig. 10). The Arg165 and Asp159 side chains interact with the ribose hydroxyl group. As noted, the density for the phosphates are weak and indeed, there are no phosphate contacts from DarR.
Analyses of a multiple DarR sequence alignment shows that the residues in the loop that contact the dinucleotides are remarkably well conserved, despite being in a region that otherwise shows significant sequence variability among homologs. In particular, residues Arg165, Trp177, Gln184 and Leu192, are completely conserved (Supplementary Fig. 5). While the strong conservation of c-di-AMP binding residues lends support to our structure, to test our structural model we mutated three of the nucleotide binding residues, Trp177, Gln184 and Leu192, to alanines and performed FP binding assays with F-c-di-AMP (Fig. 2a). These experiments showed that the DarR(W177A-Q184A-L192A) triple mutant displayed essentially no binding to c-di-AMP.
DarR binds cyclic AMP
The finding from the DarR-c-di-AMP structure that only one adenine from the dinucleotide is bound within the pocket led us to postulate that DarR might bind single adenine containing molecules. Of the adenine containing second messengers, cAMP has been shown to play a key role in mycobacterial physiology47,48,49,50,51,52,53,54,55,56,57,58,59,60,61. M. smegmatis encodes at least six putative adenylyl cyclases. Among these, MSMEG_3780 (AWT54739.1) has been shown to harbor adenylyl cyclase activity and to play a role in the acid stress response in M. smegmatis60. Both Rhodococcus sp. USK13 and M. baixiangningiae encode MSMEG_3780 homologs, WP213573200.1 and WP_193047576.1, respectively (Supplementary Fig. 11). We tested whether DarR could bind cAMP using the fluorescently labeled cAMP probe, 8-(2-[Fluoresceinyl]aminoethylthio)adenosine-3', 5'-cyclic monophosphate (F-cAMP) in FP studies. These experiments showed that Rhodococcus sp. USK13 DarR bound cAMP with a Kd of 28 ± 3 μM (Fig. 3a).
a FP isotherms showing binding of WT Rhodococcus sp. USK13 DarR (red, open circles) and Rhodococcus sp. USK13 DarR(W177A-Q184A-L192A) (blue, open squares), respectively, to F-cAMP. The x and y axes are concentration of Rhodococcus sp. USK13 DarR in μM and mP, respectively. WT Rhodococcus sp. USK13 DarR bound with a Kd of 28 ± 3 μM, while the mutant showed no binding. Data points represent mean values ± SD with the error bars centered at the mean. The error in Kd was determined as the SD between the calculated Kds for three technical replicates. b Structure of the WT Rhodococcus sp. USK13 DarR-cAMP complex. One subunit is magenta and the other green. Sigma-A weighted omit electron density (mFo-DFc) is shown as a blue mesh and contoured at 3.3 σ. c Close up of the cAMP binding-DarR interactions with omit electron density. One DarR subunit is colored green and the other, magenta. Residues that interact with cAMP are shown as sticks and labeled. d Overlay of DarR-cAMP (yellow) and the two DarR-c-di-AMP bound structures (red and slate).
We next obtained the structure of the Rhodococcus sp. USK13 DarR-cAMP complex to 1.44 Å resolution (Fig. 3b, c; Methods). The structure revealed clear density for cAMP molecules bound at each dimer interface, in the same location bound by c-di-AMP. Unlike c-di-AMP, the cAMP appears to be tightly bound to DarR; the B-factors (ave) for the cAMP is 33.5 Å2 compared to 22.6 Å2 for the protein and 36.0 Å2 for water molecules. Interestingly, in this structure the cAMP is rotated ~90 relative to the nucleotides in the c-di-AMP bound structure (Fig. 3d). Nonetheless, the contacts to the cAMP are provided by the same sets of residues that bind c-di-AMP. The Arg165 side chain contacts both the cAMP ribose and phosphate groups in the DarR-cAMP complex (Fig. 3b, c). The cAMP adenine N1 and N6 atoms are read by hydrogen bonds from Gln184 while the adenine N6 and N7 atoms are specified by the backbone carbonyl and amide nitrogen atoms of Arg179 (Fig. 3c). Finally, the side chains of DarR residues Trp177, Tyr178, Ile181 and Leu192 make hydrophobic interactions with the cAMP adenine moiety (Fig. 3c).
c-di-AMP and cAMP enhance DNA binding by DarR
To our knowledge, the cyclic adenine nucleotide binding pocket we uncovered in DarR represents a new ligand binding site for a TFR protein, separate from the DNA and inducer binding sites. This new site is located between subunits within the dimerization four-helix bundle. As all characterized TFRs bind DNA as dimers we hypothesized that dimerization stabilization by nucleotide binding at this site might facilitate DNA binding. This may be particularly critical for low DarR concentrations found in vivo. Previous studies by Zhang et al., indeed, indicated that c-di-AMP binding led to enhanced interactions with DNA, however binding affinities were not determined11. Hence, to test our hypothesis and quantify DNA binding, we used FP binding assays and determined the Kd of DarR for a 20 bp operator site in the presence of c-di-AMP and cAMP. These experiments showed that c-di-AMP and cAMP addition resulted in 2.5 and 11 fold enhancements of DNA binding; DarR bound the 20 bp operator with Kds of 4.6 ± 0.6 nM and 1.0 ± 0.2 nM in the presence of c-di-AMP and cAMP, respectively, compared to 11.4 ± 1.2 nM in the absence of these cyclic nucleotides (Supplementary Fig. 12). Hence, cAMP significantly enhances DNA binding by DarR.
DarR-DNA complexes reveal novel dimer-of-dimers-DNA interaction
Based on previous TFR-DNA structures, we presume that a dimeric form of DarR would bind to its operator site, which would explain the cyclic adenine mediated enhancement of DNA binding by DarR. However, to deduce the molecular mechanism of operator recognition by DarR, we next determined the structures of the Rhodococcus sp. USK13 and M. baixiangningiae DarR proteins in complex with a 20 bp site containing a double stranded (ds) DarR DNA operator, 5´-TAGATACTCCGGAGTATCTA-3´ (annealed to its complement). The structure of the Rhodococcus sp. USK13 DarR-DNA complex was solved first by selenomethionine SAD and refined to 2.96 Å resolution (Methods; Supplementary Table 2). This structure was then used to determine the 3.49 Å resolution structure of the M. baixiangningiae DarR-DNA complex (Fig. 4a). The Rhodococcus sp. USK13 DarR-DNA complex showed a pair of interacting DarR dimers in complex with the DNA (herein referred to as dimer-of-dimers) in the ASU while the M. baixiangningiae DarR-DNA structure contains two such complexes. These DarR-DNA complexes show the identical DNA binding mode, whereby the dimer-of-dimers partially encase the DNA using an electropositive surface (Fig. 4a, b). To assess, however, whether DarR binds DNA as a dimer-of-dimers in solution we performed size exclusion chromatography (SEC) (Fig. 4c). These analyses revealed that in the absence of DNA, the Rhodococcus sp. UK13 DarR eluted as a dimer (molecular weight (MW) of 50 kDa compared to the caculated value of 45 kDa), while, consistent with our structures, the DarR-DNA complex eluted at a MW consistent with a DarR dimer-of-dimers bound to DNA (104 kDa compared to the calculated value of 106.5 kDa) (Fig. 4c; Supplementary Fig. 13).
a Ribbon diagrams of the WT Rhodococcus sp. USK13 DarR-DNA structure and both M. baixiangningiae DarR-DNA complexes present in the crystallographic asymmetric unit (ASU) showing that the complexes adopt the same dimer-of-dimer DNA binding mode. b Electrostatic surface representation of the WT Rhodococcus sp. USK13 and M. baixiangningiae DarR-DNA complexes rotated 90° compared to Fig. 4a. c Size exclusion chromatography (SEC) analyses of apo WT Rhodococcus sp. USK13 DarR and the WT Rhodococcus sp. USK13 DarR-DNA complex. The x and y axes are Log MW and elution parameter (Kav), respectively. Elution parameter Kav calculated by Kav = (elution volume for the standard – void volume)/(column volume – void volume). Apo DarR eluted (blue oval) at a calculated molecular weight (MW) of 50 kDa, consistent with a dimer, while the DarR-DNA complex (red oval) eluted at a MW of 104 kDa consistent with a DarR dimer-of-dimers-DNA complex. The standards used for calculation of the standard curve are shown (light_blue circles) and were cytochrome c oxidase (12.4 kDa), carbonic anhydrase (29 kDa), albumin (66 kDa), alcohol dehydrogenase (150 kDa) and β-amylase (200 kDa).
The packing in the DarR-c-di-AMP structure showed that c-di-AMP bound between dimers in the crystal leading to the formation of DarR polymers (Fig. 2c), which could possibly impact DarR repression function. To investigate this possibility further we superimposed the polymers onto the DNA bound form of DarR (Supplementary Fig. 14). This overlay revealed that there would be clash from one polymer direction whilst the proteins in the other direction would extend from the DNA unimpeded. However, the extended polymer, due to the angle and distance from the DNA, would likely not have a significant impact on repression (Supplementary Fig. 14). By contrast, surface representation of the DarR DNA bound dimer-of-dimers shows that the DarR dimer-of-dimers almost completely engulfs an entire face of the DNA, which may facilitate its function as a repressing roadblock (Supplementary Fig. 15).
DarR-DarR inter-subunit contacts essential for dimer-of-dimers binding mechanism
While several TFR proteins have been shown to bind DNA as dimer-of-dimers, they have not revealed significant direct contacts between dimers27,32,33,34,35,36,38. By contrast, our DarR-DNA structures reveal that the centrally bound subunits of each of DarR dimer makes critical inter-subunit contacts (Fig. 5a; 4a, b). These β-sheet like interactions are formed between residues 116-125 of each subunit (Fig. 5a). Notably, these residues are adjacent to residues that interact with the inducer ligand in the M. baixiangningiae DarR structure and hence would be subject to allosterism (Fig. 1a). These cross subunit contacts are mediated primarily by backbone atoms whereby the amide nitrogens of Val122 and Leu120 hydrogen bond to the carbonyl oxygens of Ala116 and Leu120 of the other subunit, respectively (Fig. 5a). Further buttressing these contacts are the side chains of Arg125, which hydrogen bond to the carbonyl oxygens of Thr118 and Leu120 (Fig. 5a).
a Close-up of the interactions between the two centrally bound DarR subunits of the DarR dimer-of-dimers-DNA complex. b FP isotherms of WT Rhodococcus sp. USK13 DarR (red, open circles) and Rhodococcus sp. USK13 DarR(A119E-L120R) (blue, open squares), respectively, binding to the fluoresceinated 20 bp operator site. The x and y axis are concentration of Rhodococcus sp. USK13 DarR in nM and mP, respectively. Data points represent mean values ± SD with the error bars centered at the mean. The error in overall Kd was determined as the SD between the calculated Kds for three runs. The error in Kd was determined as the SD between calculated Kds for three experiments. c SEC analyses comparing elution of WT Rhodococcus sp. USK13 DarR and Rhodococcus sp. USK13 DarR(119E-L120R)-DNA complex. The x and y axes are LogMW and elution parameter (Kav), respectively. Kav calculated by Kav = (elution volume for the standard – void volume)/(column volume – void volume). Apo DarR eluted (blue oval) at a calculated molecular weight (MW) of 50 kDa, consistent with a dimer, while the DarR(A119E-L120R)-DNA complex (red oval) eluted at a MW of 60 kDa consistent with a DarR dimer-DNA complex. The standards used for calculation of the standard curve are the same as in Fig. 4c.
To test the importance of the DarR cross interactions to DNA binding we generated a DarR(A119E-L120R) mutant and performed biochemical experiments. This mutant was constructed based on modeling which suggested that bulky and hydrophilic substitutions in residue 119 together with residue 120 would destabilize the interface. In particular, we hypothesized that these mutations would prevent formation of the dimer-of-dimers but should still permit binding of one DarR dimer. FP analyses showed that the DarR mutant still bound DNA, but with a 5-fold reduction in affinity (mutant Kd = 59.5 ± 5 nM compared to 11.4 ± 1.2 nM for the WT). Notably, the final change in mP for the mutant was essentially half of the WT, suggesting a smaller protein mass was bound to the F-DNA by the mutant (Fig. 5b). This supported the hypothesis that the mutant may bind as a dimer. To test this hypothesis directly, we analyzed the DarR(A119E-L120R)-DNA complex by SEC. These experiments showed that the DarR(A119E-L120R)-DNA complex, indeed, eluted as a dimeric-DNA complex (MW of 61 kDa compared to the calculated MW of 60 kDa for a DarR dimer bound to DNA) (Fig. 5c; Supplementary Fig. 13).
DarR-DNA contacts
The same protein-DNA contacts are observed in the Rhodococcus sp. USK13 and M. baixiangningiae DarR-DNA structures. Hence, due to its higher resolution, we discuss DNA contacts using the Rhodococcus sp. USK13 structure (Fig. 6a, b). In the complex, the base interactions are all made to the major groove by residues from the recognition helix, α3, of each DarR subunit. One dimer docks onto the TAGATACTCC♦GGAGTATCTA palindrome, where bases that are specified by Gly45 and Lys44 are bold and underlined and the center of the palindrome is indicated by a diamond; Lys44 hydrogen bonds with the O6 of the guanine on the opposite strand of the C in the TXC motif, while Gly45 provides van der Waals interactions with the thymine methyl group (Fig. 6a–c). The close interaction between the Gly45 Cα atom and the thymine suggests any other residue at position 45 would prevent DNA interaction. Tyr48 and Tyr49 also make van der Waals interactions with thymine methyl groups in some of the subunits, but these contacts are not conserved (Fig. 6a, b). Interestingly, the second DarR dimer docks onto a DNA site, TAGATAC♦TCCGGAGTATCTA, that is pseudo-palindromic with the first site. Only one half site contains the TXC/GXA in this motif (underlined and bold) and the DarR subunit bound to this half site makes the same Lys44 and Gly45 contacts as the subunits bound to the first palindrome (Figs. 6c, 7a). Lys44 and Gly45 from the subunit bound to the non-palindromic half site do not make the base contacts observed in the other half sites (colored slate in Figs. 6a–c, 7a). As a result, this subunit is weakly bound to the DNA as underscored by poor electron density for the DNA and the bound HTH (Fig. 7a).
a Ribbon diagram showing DarR-DNA contacts. b DNA schematic showing contacts to the phosphates and bases. c Ribbon diagram showing the recognition helices of the DarR subunits bound to the DNA major groove and the locations and contacts of base interacting residues, Lys44 and Gly45. d FP isotherms for WT Rhodococcus sp. USK13 DarR binding to fluoresceinated 20 bp operator (top strand, 5´-TAGATACTCCGGAGTATCTA-3´) (red open circles), T mutant 20 bp operator (5´-TAG AGA CTC CGG CGT CTC TA-3´) (green open diamonds), G mutant 20 bp operator (5´- TAG ATA ATC CTG ATT ATC TA-3´) (blue open squares), optimized 20 bp operator (5´-TTG CTA CTC CGG AGT AT CTA-3´) (crosses). The x and y axes are concentration of Rhodococcus sp. USK13 DarR in nM and normalized D millipolarization units (mP) ((A-A0)/(Amax-A0)), respectively. A is change in mP reading, A0 is the initial mP value before addition and Amax is the maximal mP reading upon binding saturation. Normalization was done here to account for slightly different Amax values obtained for the different DNA sites. Data points represent mean values ± SD with the error bars centered at the mean. The error in Kd was determined as the SD between the calculated Kds for three technical replicate runs. e FP binding isotherms comparing the binding of WT Rhodococcus sp. USK13 DarR (red open circles), DarR(K44A) (green open diamonds) and DarR(G45V) (blue open squares) to the WT 20 bp operator. The x and y axes are concentration of Rhodococcus sp. USK13 DarR WT or mutant in nM and mP, respectively. Data points represent mean values ± SD with the error bars centered at the mean. The error in overall Kd was determined as the SD between the calculated Kds for three runs. The error in Kd was determined as the SD between the calculated Kds for the three technical replicate runs. Note, the DarR(K44A), DarR(G45V), T mutant and G mutant DNA data showed no saturable binding and hence were not fit but the points indicated with a straight line.
a Close-up of the four Rhodococcus sp. USK13 DarR recognition helices docked onto the DNA. Included is a sigma-A weighted composite 2mFo-DFc omit electron density map (contoured at 1 σ) for each bound subunit, which reveals that the density is weak for the DarR subunit bound to the non-optimized DNA site (outlined with a black box and colored slate). This DNA site does not contain the sequence that allows the Lys44-Guanine and Gly45-Thymine contacts observed in the other three sites. b Comparison of the DarR 20 bp operator site used for crystallization with the optimized operator that showed DarR enhanced binding in Fig. 6d. Each DarR subunit binds the motif, TXC where the T is contacted by Gly45 and the G on the strand opposite the C, is contacted by Lys44. The three TXC motifs in the WT operator are indicated in green, magenta and gray. Generation of the optimal site was accomplished by adding a fourth TXC motif, which is colored blue. The yellow dashed boxes indicate the motifs contacted by subunits of the first DarR dimer while the black dashed boxes correspond to the motifs bound by subunits of the second DarR dimer.
To test the DarR-DNA structural model, we analyzed DNA binding to operator sites in which the thymines and guanines in the palindrome sites were mutated. These experiments revealed that substitutions of these nucleotides prevented high affinity binding (Fig. 6d). By contrast, mutating the DNA to generate an optimized site where all four half sites contain the TXC motif led to a ∼ 2-fold enhancement in DNA binding (Kd = 6 ± 0.6 nM compared to 11 ± 1.0 nM for WT DNA) (Fig. 6d). Examination of the three characterized in vivo operator sites11 for DarR (Supplementary Fig. 16), i.e. those from the cspA, darR and Ms5347 promoters revealed that only the cspA operator site lacks a TxC half site in the second binding site (colored gray in Supplementary Fig. 16). Consistent with our structural model, EMSA studies by Zhang et al. showed that DarR bound with lower affinity to the cpsA operator site11. To further probe the structural model, we also mutated the key base specifying residues, Lys44 and Gly45 to alanine and valine residues, respectively, and showed that these mutations essentially abrogated DarR DNA binding (Fig. 6e).
In addition to base contacts, all four DarR subunits contribute phosphate contacts from the side chains of conserved residues Thr43, Tyr48, Tyr49, Ser53, Lys54 and the amide nitrogens of Ile33, Thr43 and Lys54. Lys121 from the C-terminal region that makes cross contacts also makes phosphate interactions. This residue is either a lysine or an arginine in DarR homologs, both of which could make phosphate interactions. To analyze the DarR-bound DNA conformation for unusual features that may contribute to binding we utilized the w3DNA program62. These analyses showed that while the DNA is not bent and adopts an overall B-DNA conformation (rise and twist values of 3.38 Å and 33.9° compared to 3.3 Å and 34.3° for B-DNA), the AT bases in the major groove regions bound by DarR show significant propeller twist (-10° to -29°). AT-rich DNA sites are known to exhibit high degrees of propeller twist and this may play a role in allowing the interaction of these bases with DarR residues. In addition, the major grooves of the DarR-bound DNA exhibited widening compared to B-DNA. The distance between DarR recognition helices was found to be 39 Å for both DNA bound Rhodococcus sp. USK13 and M. baixiangningiae DarR dimers. This distance is slightly larger than expected for the binding of two HTH between consecutive major grooves of B-DNA. Hence, widening of the major groove may contribute to the proper docking of DarR on operator DNA.
DarR employs a unique dual induction mechanism
The distance between HTH motifs in the DarR-DNA complex (39 Å) is similar to that between recognition helices observed in the M. smegmatis DarR structure (41 Å), supporting that the latter structure adopts a DNA-bound-like state. The distance between recognition helices in the DarR-c-di-AMP and DarR-cAMP structures is ~43 Å indicating that they also adopt structures more similar to the DNA-bound state. By contrast, the HTH distance observed in the M. baixiangningiae DarR is significantly larger, 50 Å (Fig. 1b), indicative of an induced state. Consistent with this, the M. baixiangningiae DarR structure was shown to contain a bound ligand inducer (Fig. 1a–c).
This induced structure allows us to understand the induction mechanism of DarR by comparing it with the DNA bound state. These analyses revealed that ligand binding results in a large shift in residues 108-135. This leads to a relocation of the N-terminal portion of α7, residues 121-135 and a conformational change of loop residues 117-122, compared to the DNA bound state (Fig. 8a, b). These conformational changes have two consequences. First, the shift of residues in the loop towards the dimer interface leads to a pendulum-like movement of the DNA binding domain as a unit (Fig. 8a), which leads to the large increase in the distance between DNA binding domains. Second, the structural changes in residues 117-122 prevent the formation of the cross contacts between the central subunits of the dimers in the DNA bound state, which we demonstrated is essential for the dimer-of-dimer binding mechanism (Fig. 8a). The change also removes the Lys121 contact to the DNA. Hence, DarR employs a two-part induction mechanism distinct from those of other TFR proteins.
a Comparison of M. baixiangningiae DarR-inducer (yellow) and M. baixiangningiae DarR-DNA (dark blue) complexes reveal the DarR induction mechanism. The bound inducers in the DarR-inducer complex are shown as spheres. Key points of conformational change involved in the dual induction mechanism are indicated. First, the shift in the inducer bound form upon inducer binding that would eliminate the cross contacts is noted and second, a double arrow indicates the shift that leads to an increase in the distances between the HTH in the induced form. Both structural changes would abrogate DNA binding. The pivot point identified in DynDom59, residues 108-132 are colored light_blue for the DNA-bound form and tan for the inducer bound form. b Side view of the complex with the molecular axis of rotation identified in DynDom shown as an arrow.
To investigate the induction mechanism in more detail we used DynDom59 to compare the DNA- and inducer-bound states of DarR. DynDom confirmed that there is a localized hinge bending that arises when transitioning between the two states (Fig. 8b). Consistent with our analyses, DynDom59 calculated that the rotation axis in transiting from one state to the next is composed of residues 108-132. This transition is accompanied by a rotation of 19.7 ° and a small translation of 0.2 Å, of the DNA-binding domains relative to the C-terminal domain. This again is a two component mechanism as in addition to repositioning of the DNA-binding domains, the movement of residues within the hinge region leads to a conformational change that prevents cross contacts.
Discussion
TFR proteins are among the best characterized one component regulators from biological and structural standpoints. Structures of TFR proteins reveal that they share a two-domain architecture with an N-terminal DNA-binding domain and a C-terminal inducer binding/dimerization domain. Although numerous TFR structures have been determined, comparatively few have been solved in both inducer- and DNA-bound forms. Such studies are complicated by the fact that the identification of inducers is not currently predictable and hence requires experimentation. DarR, the N-terminal sequence of which places it within the TFR family, was discovered via an unusual route, which was based on its ability to bind c-di-AMP11.
The finding that a TFR bound c-di-AMP was of interest as were data indicating that it did not appear to function as an inducer. These data suggested that DarR might bind the nucleotide using a newly described mechanism. This was confirmed by our DarR-c-di-AMP structure, which showed the second messenger does not bind within the canonical inducer binding pocket nor the DNA binding domain, but within the C-terminal four helix bundle region. Hence, the structure expands the known ligand binding/allosteric sites of TFR proteins. Studies have started to shed light on the molecular mechanisms utilized by c-di-GMP, and to a lesser extent, c-di-AMP, binding receptors. These analyses have shown that most c-di-GMP binding signatures are characterized by the presence of arginines and aspartic acids, with RxxD being the most observed c-di-GMP binding motif12,13,14,15. Structures of c-di-AMP bound receptors display a larger range of binding mechanisms, involving hydrogen bonds from backbone atoms and the side chains of asparagine, threonine and glutamic acid20. Arguably, the best-studied c-di-AMP binding motif is the regulator of conductance of K+ (RCK) domain63,64,65, which is found in multiple potassium channels and transporters63. The RCK domain consists of an antiparallel β-sheet with a central, c-di-AMP interacting helix and c-di-AMP binds at the center of the dimer63,64,65. The RCK motif was also found to bind c-di-AMP in the recently characterized BusR transcription regulator64. However, the c-di-AMP binding site revealed in DarR shows no homology to any of these characterized c-di-GMP or c-di-AMP binding motifs.
Interestingly, our DarR-c-di-AMP structure showed one adenine moiety was primarily contacted by DarR with the other, solvent exposed. This led us to hypothesize and then demonstrate that DarR binds cAMP. The mid-μM binding affinity of DarR for cAMP suggests this as a functionally important second messenger as intracellular concentrations of cAMP in M. smegmatis have been estimated to reach levels up to ~3 mM60. By contrast, c-di-AMP levels in M. smegmatis appear to be in the nM range61. Studies have shown that high target specificity for second messengers can be achieved with local signaling events between specific cyclases and target proteins66. However, the large difference in concentrations of the second messengers in M. smegmatis as well as the significant enhancement in DNA binding afforded by cAMP, would point to cAMP as a more likely physiological ligand for DarR.
As a central signaling molecule in prokaryotes as well as eukaryotes, cAMP has been the subject of intense study47,48,49,50,51,52,53,54,55,56,57,58. Structural analyses have revealed two common cAMP/cGMP binding motifs, the nucleotide monophosphate binding domain (CNB) and the GAF motif, which was named after the cGMP-specific phosphodiesterases, adenylyl cyclases and FhlA57. The CNB, which binds both cAMP and cGMP, is composed of a β-barrel surrounded by three α helices and is found in bacterial as well as eukaryotic proteins. Structures solved of CNB containing proteins include members of the bacterial CRP-FNR transcription factors and the regulatory subunit of the cAMP-dependent protein kinase in eukaryotes47,48,49,50,51,52,53,54,55,56,57,58. The GAF motif was only recently described and is comprised of two β-strands and α helix57. The cAMP binding region in DarR shows no homology to either of these motifs and instead contains a W(X)6Q(X)7L signature and includes an arginine from the adjacent subunit. Thus, this motif consists of few conserved residues making it difficult to identify other receptors containing this signature. However, sequence analyses show that it is present in all or most DarR proteins (Supplementary Fig. 3).
Because cAMP interact with residues from both subunits of the DarR dimer, it would be predicted to function as a stabilizer of dimerization. Unfortunately, the oligomerization status of TFRs, including DarR, at the low concentrations found in cells remains largely unexplored. Indeed, solution biochemical studies, such as SEC, assessing the oligomeric states of TFRs tend to be performed at high protein concentrations that would favor dimers. Our DNA binding assays, which were carried out in the nM range, more consistent with physiological conditions, showed the cyclic adenine nucleotides enhanced DNA binding, consistent with a dimer stabilizing role. Strikingly, when we analyzed TFR structures present in the Protein Data Bank, we noticed that these proteins can be categorized into two main groups based on the different types of dimerization modules. The most abundant category, which also includes DarR, are comprised of a C-terminal region with two helices, (α8 and α9 in DarR) that combine to generate a simple four helix bundle dimerization module. A second category includes members that have extra structural elements inserted between α8 and α9 that, notably, contribute to dimerization. In particular, there are several TFR proteins that contain 2 extra helices within this region that participate in extensive dimer contacts (Supplementary Fig. 17). The binding of a small molecule, such as cAMP, that facilitates dimerization, such as in DarR, may be a way to shore up dimerization in TFR members that lack such dimer stabilizing elements.
In conclusion, our combined data have uncovered a previously unknown allosteric ligand binding site for a TFR. Our DarR-DNA and DarR-effector structures also reveal a unique dimer-of-dimers DNA binding mode and induction mechanism for a TFR protein, that involves intimate cross dimer contacts on the DNA. These combined studies thus indicate that despite the extensive structural and biological characterizations carried out on multiple TFRs, much remains to be discovered about the ligand and DNA binding mechanisms of these proteins.
Methods
Purification of DarR proteins
Genes encoding the M. smegmatis DarR, Rhodococcus sp. USK13 DarR and M. baixiangningiae DarR proteins were purchased as codon optimized genes (for E. coli expression) from Genscript (Piscataway, NJ, USA; http://www.genscript.com). The genes were subcloned into NdeI/BamHI sites in pET15b, which resulted in expressed proteins with a cleavable, N-terminal Hexa-histidine tag (His-tag). E. coli C41(DE3) cells were transformed with these pET15b vectors. For expression of DarR proteins, cells were grown to an OD600 of 0.6 and induced with 1.0 mM IPTG overnight at 15 °C. Cells were reconstituted into buffer A (25 mM Tris-HCl pH 7.5, 300 mM NaCl, 5% (v/v) glycerol, 0.5 mM β-mercaptoethanol (β-ME)) and lysed using a microfluidizer or sonicator. Cell debris was removed by centrifugation at 40,000 x g. The lysate, which contained the soluble DarR proteins, was loaded onto a NTA-Cobalt column and the column was washed with 500 mL of buffer A containing 15 mM imidazole. The protein was eluted in steps of increasing imidazole from 20 mM to 300 mM and fractions containing the protein were combined. At this stage the protein was >95% pure as assessed by SDS PAGE. Mutant DarR proteins were expressed and purified using the same protocol.
DNA binding assays
To measure DNA binding to DarR, 5' fluoresceinated DNA (F-DNA) sites were used. For these experiments a buffer consisting of 25 mM N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) pH 7.5, 150 mM NaCl and 5% (v/v) glycerol was used. All the FP DNA binding experiments used F-DNA probe at a final concentration of 1 nM. For each experiment, increasing concentrations of DarR (WT or mutant) were titrated into the reaction cell. To assess the impact of c-di-AMP and cAMP on DNA binding, the adenine nucleotides were present in the buffer and protein solutions at a concentration of 1 mM. All FP experiments were conducted at 25 °C and performed in triplicate. The resultant data were plotted using KaleidaGraph to deduce binding affinities.
c-di-AMP binding experiments
To measure c-di-AMP binding to DarR or DarR mutants, 2′-O-(6-[Fluoresceinyl]aminohexylcarbamoyl)-cyclic diadenosine monophosphate (2′-Fluo-AHC-c-di-AMP) (BioLog), was used as a fluoresceinated reporter ligand. This molecule is conjugated via a nine atom spacer to one of the c-di-AMP 2′ hydroxyl groups. The structure of the DarR-c-di-AMP complex showed that one ribose hydroxyl in the bound c-di-AMP is solvent exposed indicating it should bind DarR. These experiments were performed in a buffer consisting of 25 mM HEPES pH 7.5, 150 mM NaCl, 5% (v/v) glycerol and contained 1 nM 2′-Fluo-AHC-c-di-AMP. All FP experiments were conducted at 25 °C and performed in triplicate. The resultant data were plotted using KaleidaGraph to deduce binding affinities.
cAMP binding experiments
To measure cAMP binding to DarR, 2′-O-(6-[Fluoresceinyl]aminohexylcarbamoyl)-cyclic diadenosine monophosphate (8-(2- [Fluoresceinyl]aminoethylthio)adenosine- 3', 5'- cyclic monophosphate (8-[Fluo]-cAMP) (Axxora), was used as a fluoresceinated probe. This molecule contains a fluorescein attached to the C8 atom of the adenosine ring, which is solvent exposed in the structure of DarR with cAMP. FP binding experiments were carried out in a buffer consisting of 25 mM HEPES pH 7.5, 150 mM NaCl, 5% (v/v) glycerol and contained 1 nM 8-[Fluo]-cAMP. All FP experiments were conducted at 25 °C and performed in triplicate. The resultant data were plotted using KaleidaGraph to deduce binding affinities.
Size exclusion chromatography (SEC) experiments
SEC studies were performed using a SUPERDEXTM 200 pg HiloadTM 26/600 column and an AKTA prime plus. The buffer used for the runs was 25 mM HEPES pH 7.5, 150 mM NaCl, 5% (v/v) glycerol and 0.5 mM βME. Fractions were concentrated using Sigma-Millipore concentrators (Amicon) prior to column application. Samples were loaded using a 1 mL (final volume) syringe. The SEC studies were carried out on apo Rhodococcus sp. USK13 DarR (at 500 μM, per monomer), the WT Rhodococcus sp. USK13 DarR complex with ds 20 bp DarR operator DNA (top strand, 5´-TAGATACTCCGGAGTATCTA-3´ annealed to its complement) and DarR(L119R-A120E) bound to the 20mer. The WT DarR-DNA complex used 200 μM DarR (concentration of the monomer) to 500 μM dsDNA, the DarR(L119R-A120E)-DNA complex used 150 μM monomer protein to 350 μM dsDNA. The elution volumes of each sample were compared with a series of protein standards to determine the molecular weights. The standards used for calculation of the standard curve were cytochrome c (12.4 kDa), carbonic anhydrase (29.0 kDa), bovine serum albumin (66.0 kDa), alcohol dehydrogenase (150.0 kDa) and β-amylase (200 kDa).
Crystallization and structure determination of apo M. smegmatis DarR and M. baixiangningiae DarR
For crystallization, the N-terminal His-tags of M. smegmatis DarR and M. baixiangningiae DarR were removed using a thrombin cleavage capture kit (EMD Millipore). The tag free proteins were then concentrated to 7 mg/mL (M. smegmatis DarR) and 30 mg/mL (M. baixiangningiae DarR) and used for screening using the hanging drop vapor diffusion method at room temperature (rt) with Wizard I-IV, Salt Rx 1 and 2, PEG Rx 1 and 2 and Natrix screens. Small crystals were identified and conditions optimized. The final crystals used for data collection of the M. smegmatis DarR were obtained by mixing the protein 1 to 1 with a crystallization solution of 22% (w/v) PEG 3350 and 0.1 M Citrate pH 5.6. These crystals grew within a few days and reached maximum size after 2 weeks. Optimal crystals of M. baixiangningiae DarR were obtained by mixing the protein 1 to 1 with a crystallization solution consisting of 2.5 M NaCl, 0.1 M Tris pH 8.5. Crystals grew after 2–5 days and continued to grow for 1 week. The M. smegmatis DarR and M. baixiangningiae DarR crystals were cryo-preserved by dipping them in a drop containing the reservoir supplemented with 20% (v/v) glycerol for 2–3 s (s) before plunging into liquid nitrogen. Data were collected at the Advanced Light Source (ALS) beamline 5.0.2 and processed with XDS (version January10, 2022)67. Native data sets were collected to 3.56 Å and 1.9 Å for the M. smegmatis DarR and M. baixiangningiae DarR crystals, respectively.
To solve the phase problem, selenomethionine-substituted M. baixiangningiae DarR was produced and used to grow crystals for single wavelength anomalous diffraction (SAD) experiments. The selenomethionine-substituted protein was expressed and purified as per the WT. After cleavage of the His-tag and concentration to 30 mg/mL, the selenomet-M. baixiangningiae DarR was crystallized using the same protocol as for the WT protein. The crystals were cryo-preserved as per the WT crystals and SAD data collected at ALS beamline 5.0.2 to 1.6 Å resolution. Due to the higher resolution, the selenomethionine DarR data was used in final model building and refinement. The WT structure (1.9 Å) was identical to the Semet structure. Phenix Autosol (using Phenix version 1.20.1-4487) was used to located selenium sites, perform phasing and carry out density modification68. The resultant experimental SAD map permitted autotracing of 80% of the structure, which contains one subunit in the asymmetric unit (ASU) in Coot (version 0.8.9.2)69. Crystallographic symmetry generates the DarR dimer. After correcting regions that were improperly fit and building regions that had not been autotraced, the model was further refined. At this point there was clear density in the binding pocket that had an unusual spirocyclic, ring shaped structure. After fitting individual carbon and oxygen atoms into the density, it became clear that the best fit and the one with the most chemical sense included a glycerol molecule coordinated with Tris and boron. The eLBOW software within Phenix68 was used to generate geometry restraint information of the borate complex ligand. After inclusion of the ligand, the Rfree dropped by 0.5%. Finally, solvent molecules were added, and the structure refined to convergence.
The M. smegmatis structure contains two dimers in the ASU and the structure was solved using a M. baixiangningiae DarR subunit as the search model in Phenix68. Four solutions were obtained, which generated the two M. smegmatis dimers. After one round of refinement in Phenix, the side chains that differ in the two proteins were replaced with the M. smegmatis sequence and the model refined further in Phenix68. One dimer, in particular its DNA binding domains, had poor density, likely explaining the slightly elevated Rfree. After multiple rounds of rebuilding in Coot and refinement in Phenix68, the M. smegmatis DarR model converged to Rwork/Rfree values of 27.5%/30.5% to 3.56 Å resolution. Final data collection and refinement statistics are presented in Supplementary Table 1.
Crystallization and structure determination of Rhodococcus sp. USK13 DarR with c-di-AMP
For crystallization trials of Rhodococcus sp. USK13 DarR with c-di-AMP, the N-terminal His-tag of DarR was removed using a thrombin cleavage capture kit. For crystallization trials, the protein (at 30 mg/mL) was mixed with a final concentration of 5 mM c-di-AMP (Sigma-Aldrich; SML1231). Hanging drop vapor diffusion crystallization trials of the complex were performed at rt. Large rod like crystals were generated by mixing the complex 1 to 1 with 0.1 M Tris pH 8.5, 1.34 M lithium sulfate and 1.3% (v/v) PEG 400. Crystals grew in 2–3 days and reached maximum size in a week. The crystals were cryo-preserved by dipping them in the crystallization solution supplemented with 25% (v/v) glycerol for 2–3 s before plunging into liquid nitrogen. Data were collected at ALS beamline 5.0.1 and the structure was solved using a dimer of the M. baixiangningiae DarR structure as a search model. Two dimers are in the ASU and were readily placed in MR using Phenix68. Following an initial round of Phenix_refine68, the side chains that differ between the two DarR proteins were replaced with those in Rhodococcus sp. USK13 DarR. After several rounds of refitting and refinement, clear density was observed for a di-adenine-nucleotide, which was fitted. Following water addition, the structure was refined to final Rwork/Rfree of 21.7%/25.9% to 2.45 A resolution (Supplementary Table 1).
Crystallization and structure determination of Rhodococcus sp. USK13 DarR with cAMP
Rhodococcus sp. USK13 DarR in which the N-terminal His-tag was removed was used for crystallization trials with cAMP by mixing the protein (at 30-35 mg/mL) with 2 mM cAMP (Sigma-Aldrich; A6885). The mixture was used in hanging drop vapor diffusion experiments at rt. Purified Rhodococcus sp. USK13 DarR(K44A) with the His-tag removed was also used to generate crystals. This mutant was produced to test effects on DNA binding (the mutation is located in the DNA binding domain, far from the cAMP binding site) and was used here as a surface entropy reduction mutant70 to assess if crystals of the mutant in complex with cAMP could be more readily obtained. Indeed, the mutant produced larger crystals of DarR in complex with cAMP, grown under the same conditions as WT, allowing for the collection of data to 1.44 Å resolution. Optimal crystals were produced using 800 mM succinic acid pH 7.0 as a crystallization solution. The crystals were cryopreserved by dipping them in a solution consisting of the crystallization reagent supplemented with 20% (v/v) glycerol for 1–2 s before plunging into liquid nitrogen. Data were collected at ALS beamline 5.0.2 and the structure was solved using a monomer of the Rhodococcus sp. USK13 DarR structure (from the c-di-AMP bound structure) as a search model. Crystallographic symmetry generates the DarR dimer. Following an initial round of Phenix_refine68, density was evident for a cAMP molecule, which was fitted. After several rounds of refinement68 and water addition, the structure was refined to convergence (Supplementary Table 1).
Crystallization and structure determination of Rhodococcus sp. USK13 DarR-DNA complex
For crystallization of Rhodococcus sp. USK13 DarR with DNA, tag-free Rhodococcus sp. USK13 DarR at 30 mg/mL was mixed with 20 bp operator DNA, 5´-TAGATACTCCGGAGTATCTA-3´ (top strand annealed to its complement to generate ds blunt ended DNA) (1:1 dimer: DNA duplex) and utilized in crystallization screens using hanging drop vapor diffusion at rt. Crystals were obtained by mixing the complex 1 to 1 with solutions containing 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES) pH 6.5, 30% (w/v) PEG 8000 and 0.1 M calcium acetate. Crystals grew as long rods and took 2–3 weeks to reach maximum size and were cryopreserved by dipping them in a drop of the crystallization solution supplemented with 25% (v/v) ethylene glycol. Data were collected at ALS beamline 5.0.1 and processed with XDS67. The structure was solved by selenomethionine SAD using data collected from a crystal grown with selenomet-substituted Rhodococcus sp. USK13 DarR bound to the 20 bp DNA site. Selenomethionine sites were identified and refined and density modification was performed in Phenix AutoSol68. While autotracing was not successful due to the low resolution, the map could be manually traced in Coot69. The DNA register was defined by the weaker density of the DarR subunit bound at the end of the DNA duplex with the nonoptimal site (see Results). Final data collection and refinement statistics are provided in Supplementary Table 2.
Crystallization and structure determination of M. baixiangningiae DarR-DNA complex
For crystallization of M. baixiangningiae DarR with DNA, tag-free protein at 20 mg/mL was mixed with the 20 bp operator DNA, 5´-TAGATACTCCGGAGTATCTA-3´ (top strand annealed to its complement to generate blunt ended ds DNA) (1:1 dimer: DNA duplex). Crystallization screens were carried out using the hanging drop vapor diffusion method at rt. Crystals were obtained by mixing the complex 1:1 with solutions containing 0.1 M sodium citrate tribasic dihydrate pH 5.0, 0.2 M MgCl2 and 13% (w/v) PEG 20,000. Crystals took 1–2 weeks to grow. The crystals were cryopreserved by dipping them in a drop of the crystallization solution supplemented with 25% (v/v) ethylene glycol before plunging them in liquid nitrogen. Data were collected at ALS beamline 5.0.1 and processed with XDS67. To solve the structure, the Rhodococcus sp. USK13 DarR-DNA structure was used as a search model. There are two dimer-of-dimer M. baixiangningiae DarR-DNA complexes in the ASU, which were successfully located in MR. After a round of refinement in Phenix_refine, the side chains were replaced for those in the M. baixiangningiae DarR protein. The DNA register of one complex was clearly defined by the weaker density of the DarR subunit bound at the DNA end with the nonoptimal site. The second complex was less clear and DNA register was determined by trying both possibilities with one selected based on the lower Rfree after refinement. Final data collection and refinement statistics are included in Supplementary Table 2.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in the article. Coordinates and structure factor amplitudes for the structures have been deposited in the RCSB Protein Data Bank under the accession codes 8SV6, 8SUA, 8SUK, 8T5Y, 8SVA and 8SVD. Other source data are provided as a Source_data file. Source data are provided with this paper.
References
Cuthbertson, L. & Nodwell, F. R. The TetR family of regulators. MMBR 77, 440–475 (2013).
Ramos, J. L. et al. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69, 326–356 (2005).
Ulrich, L. E., Koonin, E. V. & Zhulin, I. B. One-component systems dominate signal transduction in prokaryotes. Trends Microbiol 5, 710–720 (2005).
Deng, W., Li, C. & Xie, J. The underlying mechanism of bacterial TetR/AcrR family transcriptional repressors. Cell Signal 25, 1608–1613 (2013).
Izaki, K., Kiuchi, K. & Arima, K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J. Bacteriol. 91, 628–633 (1966).
Postle, K., Nguyen, T. T. & Bertrand, K. P. Nucleotide sequence of the repressor gene of the Tn10 tetracycline resistance determinant. Nucleic Acids Res 12, 4849–4863 (1984).
Wray, L. V., Jorgensen, R. A. & Reznikoff, W. S. Identification of the tetracycline resistance promoter and repressor in transposon Tn10. J. Bacteriol. 147, 297–304 (1981).
Kisker, C., Hinrichs, W., Tovar, K., Hillen, W. & Saenger, W. The complex formed between Tet repressor and tetracycline-Mg2+ reveals mechanism of antibiotic resistance. J. Mol. Biol. 247, 260–280 (1985).
Ahn, S. K., Cuthbertson, L. & Nodwell, J. R. Genome context as a predictive tool for identifying regulatory targets of the TetR family transcriptional regulators. PLoS One 7, e50562 (2012).
Yu, Z., Reichheld, S. E., Savchenko, A., Parkinson, J. & Davidson, A. R. A comprehensive analysis of structural and sequence conservation in the TetR family transcriptional regulators. J. Mol. Biol. 400, 847–864 (2010).
Zhang, L., Li, W. & He, Z.-G. DarR, a TetR-like transcriptional factor, is a cyclic-di-AMP responsive repressor in Mycobacterium smegmatis. J. Biol. Chem. 288, 3085–3096 (2012).
Jenal, U., Reinders, A. & Lori, C. Cyclic di-GMP, second messenger extraordinaire. Nat. Rev. Microbiol. 15, 271–284 (2017).
Hengge, R. Principles of c-di-GMP signaling in bacteria. Nat. Rev. Microbiol. 7, 263–273 (2009).
Valentini, M. & Filloux, A. Multiple roles of c-di-GMP signaling in bacterial pathogenesis. Annu. Rev. Microbiol. 73, 387–406 (2019).
Wang, Z., Song, L., Liu, Z., Shen, Z. & Li, X. Bacterial second messenger c-di-GMP, emerging functions in stress resistance. Microbiol. Res. 268, 127302 (2023).
Bush, M. J., Tschowri, N., Schlimpert, S., Flärdh, K. & Buttner, M. J. c-di-GMP signalling and the regulation of developmental transitions in streptomycetes. Nat. Rev. Microbiol. 13, 749–760 (2015).
Stülke, J. & Krüger, L. Cyclic di-AMP signaling in bacteria. Annu. Rev. Microbiol. 8, 159–179 (2020).
Zarella, T. M. & Bai, G. The many roles of the bacterial second messenger cyclic di-AMP in adapting to stress cues. J. Bacteriol. 203, e00348–20 (2021).
Corrigan, R. M. & Gründling, A. Cyclic di-AMP, another second messenger enters the fray. Nat. Rev. Microbiol. 11, 513–524 (2013).
He, J., Yin, W., Galperin, M. Y. & Chou, S.-H. Cyclic di-AMP, a second messenger of primary importance, tertiary structures and binding mechanisms. Nucleic Acids Res 48, 2807–2829 (2020).
Witte, G., Hartung, S., Büttner, K. & Hopfner, K. P. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell 30, 167–178 (2008).
Liu, S., Bayles, D. O., Mason, T. M. & Wilkinson, B. J. A cold-sensitive Listeria monocytogenes mutant has a transposon insertion in a gene encoding a putative membrane protein and shows altered (p)ppGpp levels. Appl. Environ. Microbiol. 72, 3955–3959 (2006).
Arguedas-Villa, C., Stephan, R. & Tasara, T. Evaluation of cold growth and related gene transcription responses associated with Listeria monocytogenes strains of different origins. Food Microbiol 27, 653–660 (2010).
Beggs, G. A. et al. Structures of Neisseria gonorrhoeae MtrR-operator complexes reveal molecular mechanisms of DNA recognition and antibiotic resistance-conferring clinical mutations. Nucleic Acids Res 49, 4155–4170 (2021).
Le, T. B. K., Schumacher, M. A., Lawson, D. M., Brennan, R. G. & Buttner, M. J. The crystal structure of the TetR family transcriptional repressor SimR bound to DNA and the role of a flexible N-terminal extension in minor groove binding. Nucleic Acids Res 39, 9433–9437 (2011).
Miller, D. J., Zhang, Y. M., Subramanian, C., Rock, C. O. & White, S. W. Structural basis for the transcriptional regulation of membrane lipid homeostasis. Nat. Struct. Mol. Biol. 17, 971–975 (2010).
Schumacher, M. A. et al. Structural basis for the cooperative DNA binding by two dimers of the multidrug binding protein QacR. EMBO J. 21, 1210–1218 (2002).
Sawai, H., Yamanaka, M., Sugimoto, H., Shiro, Y. & Aono, S. Structural basis for the transcriptional regulation of heme homeostasis in Lactococcus lactis. J. Biol. Chem. 287, 30755–30768 (2012).
Bock, T. et al. The AibR-isovaleryl coenzyme A regulator and its DNA binding site- a model for the regulation of alternative de novo isovaleryl coenzyme A biosynthesis in Myxococcus xanthus. Nucleic Acids Res 45, 2166–2178 (2017).
Schumacher, M. A. et al. Structural mechanisms of QacR induction and multidrug recognition. Science 294, 2158–2163 (2001).
Orth, P., Schnappinger, D., Hillen, W., Saenger, W. & Hinrichs, W. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat. Struct. Mol. Biol. 7, 215–219 (2000).
Yang, S. F. et al. Structural basis for interaction between Mycobacterium smegmatis Ms6564, a TetR family master regulator, and its target DNA. J. Biol. Chem. 288, 23687–23695 (2013).
Bhukya, H., Jana, A. K., Sengupta, N. & Anand, R. Structural and dynamics studies of the TetR family protein, CprB from Streptomyces coelicolor in complex with its biological operator sequence. J. Struct. Biol. 198, 134–146 (2017).
Kim, Y., Kang, Y. & Choe, J. Crystal structure of Pseudomonas aeruginosa transcriptional regulator PA2196 bound to its operator DNA. Biochem. Biophys. Res. Commun. 440, 317–321 (2013).
Tonthat, N. K. et al. SlmA forms a higher-order structure on DNA that inhibits cytokinetic Z-ring formation over the nucleoid. Proc. Natl Acad. Sci. USA 110, 10586–10591 (2013).
Itou, H., Watanabe, N., Yao, M., Shirakihara, Y. & Tanaka, I. Crystal structures of the multidrug binding repressor Corynebacterium glutamicum CgmR in complex with inducers and with an operator. J. Mol. Biol. 403, 174–184 (2010).
Palanca, C. & Rubio, V. Structure of AmtR, the global nitrogen regulator of Corynebacterium glutamicum, in free and DNA-bound forms. FEBS J. 283, 1039–1059 (2016).
Schumacher, M. A. & Zeng, W. Structures of the nucleoid occlusion protein SlmA bound to DNA and the C-terminal domain of the cytoskeletal protein FtsZ. Proc. Natl Acad. Sci. USA 113, 4988–4993 (2016).
Colclough, A. L., Scadden, J. & Blair, J. M. A. TetR-family transcription factors in Gram-negative bacteria, conservation, variation and implications for efflux-mediated microbial resistance. BMC Genom. 20, 731 (2019).
Lee, J. Y., Yeo, H. K. & Park, T. W. Structural basis of operator sites recognition and effector binding in the TetR family transcription regulator FadR. Nucleic Acids Res 45, 4244–4254 (2017).
Yan, L. et al. Structural insights into operator recognition by BioQ in the Mycobacterium smegmatis biotin synthesis pathway. Biochim. Biophys. Acta 1862, 1843–1851 (2018).
Lara, J. et al. Mycobacterium tuberculosis FasR senses long fatty acyl-CoA through a tunnel and a hydrophobic transmission spine. Nat. Commun. 11, 3703 (2020).
Agari, Y., Sakamoto, K., Kuramitsu, S. & Shinkai, A. Transcriptional repression mediated by a TetR family protein, PfmR, from Thermus thermophilus HB8. J. Bacteriol. 194, 4630–4641 (2012).
Cesselin, B. et al. Discovery of intracellular heme-binding protein HrtR, which controls heme efflux by the conserved HrtB-HrtA transporter in Lactococcus lactis. J. Biol. Chem. 287, 4752–4758 (2012).
Rezanka, T. & Sigler, K. Biologically active compounds. Stud. Nat. Prod. Chem. 35, 835–921 (2008).
Coddington, J. M. & Taylor, M. J. High field 11B and 13C NMR investigations of aqueous borate solutions and borate-thiol complexes. J. Coord. Chem. 20, 27–38 (1989).
Knapp, G. S. & McDonough, K. A. Cyclic AMP signaling in Mycobacteria. Microbiol. Spectr. 2, MGM2-0011-2013 (2014).
Bai, G., Knapp, G. S. & McDonough, K. A. Cyclic AMP signaling in mycobacteria, redirecting the conversation with a common currency. Cell Microbiol 12, 349–358 (2010).
Lee, C. H. Identification of adenosine 3’,5’-monophosphate in Mycobacterium smegmatis. J. Bacteriol. 132, 1031–1033 (1997).
Wong, A. I. et al. Cyclic AMP is a critical mediator of intrinsic drug resistance and fatty acid metabolism in M. tuberculosis. eLife 12, e81177 (2023).
Khan, H. et al. Convergence of two global regulators to coordinate expression of essential virulence determinants of Mycobacterium tuberculosis. eLife 11, 10.7554 (2022).
Dremier, S., Kopperud, R., Doskeland, S. O., Dumont, J. E. & Maenhaut, C. Search for new cyclic AMP-binding proteins. FEBS Lett. 546, 103–107 (2003).
Yarwood, S. J. Special issue on “New advances in cyclic AMP signalling”- an editorial overview. Cells 9, 2274 (2020).
Banerjee, A. et al. A universal stress protein (USP) in Mycobacteria bind cAMP. J. Biol. Chem. 290, 12731–12743 (2015).
Rehmann, H. et al. Structure and regulation of the cAMP-binding domains of Epac2. Nat. Struct. Mol. Biol. 10, 26–32 (2003).
Berman, H. M., Ten Eyck, L. F., Goodsell, D. S. & Taylor, S. S. The cAMP binding domain, an ancient signaling module. Proc. Natl Acad. Sci. USA 102, 45–50 (2004).
Zoraghi, R., Corbin, J. D. & Francis, S. H. Properties and functions of GAF domains in cyclic nucleotide phosphodiesterases and other proteins. Mol. Pharmacol. 65, 267–278 (2004).
Dass, B. K., Sharma, R., Shenoy, A. R., Mattoo, R. & Visweswariah, S. S. Cyclic AMP in mycobacteria, characterization and functional role of the Rv1647 ortholog in Mycobacterium smegmatis. J. Bacteriol. 190, 3824–3834 (2008).
Veevers, R. & Hayward, S. Methodological improvements for the analysis of domain movements in large biomolecular complexes. Biophys. Physicobiol. 16, 328–336 (2019).
Dass, B. K. M., Sharma, R., Shenoy, A., Mattoo, R. & Visweswariah, S. S.Cyclic AMP in Mycobacteria: characterization and function of the Rv1647 ortholog in Mycobacterium smegmatis.J. Bacteriol. 190, 3824–3834 (2008).
Ning, H. et al. c-di-AMP accumulation regulates growth, metabolism, and immunogenicity of Mycobacterium smegmatis. Font. Microbiol. 13, 865045 (2022).
Li, S., Olson, W. K. & Lu, X.-J. Web 3DNA for the analysis, visualization, and modeling of 3D nucleic acid structures. Nucleic Acids Res 47, W26–W34 (2019).
Cereija, T. B., Guerra, J. P. L., Jorge, J. M. P. & Morais-Cabral, J. H. c-di-AMP, a likely master regulator of bacterial K+ homeostasis machinery, activates a K+ exporter. Proc. Natl Acad. Sci. USA 118, e20020653118 (2021).
Bandera, A. M. et al. BusR senses bipartite DNA binding motifs by a unique molecular ruler architecture. Nucleic Acids Res 49, 10166–10177 (2021).
Corrigan, R. M. et al. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc. Natl Acad. Sci. USA 110, 9084–9089 (2013).
Hengge, R. High-specificity local and global c-di-GMP signaling. Trends Microbiol 29, 993–1003 (2021).
Kabsch, W. XDS. Acta Crystallogr. D. Biol. Crystallogr. 66, 125–132 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons, recent developments in Phenix. Acta Cryst. D75, 861–877 (2019).
Emsley, P. & Cowtan, K. Coot, model-building tools for molecular graphics. Acta Cryst. D60, 2126–2132 (2004).
Cooper, D. R. et al. Protein crystallization by surface entropy reduction, optimization of the SER strategy. Acta Crystallogr. D. Biol. Crystallogr. 63, 646–645 (2007).
Acknowledgements
This research was supported by National Institutes of Health grants (R35-GM130290 and a Nanaline H Duke Professor of Biochemistry Award to M.A.S.). We acknowledge beamline 5.0.1 and 5.0.2 for X-ray diffraction data collection. The ALS (Berkeley, CA) is a national user facility operated by Lawrence Berkeley National Laboratory on behalf of the US Department of Energy under Contract DE-AC02-05CH11231, Office of Basic Energy Sciences. Beamline 5.0.2 of the ALS, a US Department of Energy Office of Science User Facility under Contract DE-AC02-05CH11231, is supported in part by the ALS-ENABLE program funded by the NIH, National Institute of General Medical Sciences, Grant P30 GM124169-01. We would like to acknowledge Dr. Kenichi Yokoyama for providing important insight into the Tris-B-glycerol ligand. We also thank Professor Richard G. Brennan for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
M.A.S. and R.S. participated in experimental design. N.L. and R.S. made samples for structural and biochemical analyses. R.S. performed SEC experiments and performed SEC analyses. V.C. aided in determination of the Tris-B-glycerol ligand. M.A.S. performed FP analyses, grew crystals, and solved the crystal structures. M.A.S. performed analyses and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shekhar Mande, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schumacher, M.A., Lent, N., Chen, V.B. et al. Structures of the DarR transcription regulator reveal unique modes of second messenger and DNA binding. Nat Commun 14, 7239 (2023). https://doi.org/10.1038/s41467-023-42823-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42823-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.