Abstract
Understanding methane (CH4) emission from thermokarst lakes is crucial for predicting the impacts of abrupt thaw on the permafrost carbon-climate feedback. However, observational evidence, especially from high-altitude permafrost regions, is still scarce. Here, by combining field surveys, radio- and stable-carbon isotopic analyses, and metagenomic sequencing, we present multiple characteristics of CH4 emissions from 120 thermokarst lakes in 30 clusters along a 1100 km transect on the Tibetan Plateau. We find that thermokarst lakes have high CH4 emissions during the ice-free period (13.4 ± 1.5 mmol m−2 d−1; mean ± standard error) across this alpine permafrost region. Ebullition constitutes 84% of CH4 emissions, which are fueled primarily by young carbon decomposition through the hydrogenotrophic pathway. The relative abundances of methanogenic genes correspond to the observed CH4 fluxes. Overall, multiple parameters obtained in this study provide benchmarks for better predicting the strength of permafrost carbon-climate feedback in high-altitude permafrost regions.
Similar content being viewed by others
Introduction
Soils in high-latitude and high-altitude permafrost regions store more than 50% of world’s soil organic carbon (SOC) in about 15% of the global land area1,2 and play an important role in the global carbon cycle. These permafrost regions are experiencing higher rates of climate warming compared to other parts of the world3. This rapid warming is triggering widespread gradual and abrupt permafrost thaw4 and subsequent release of carbon to the atmosphere in the form of carbon dioxide (CO2) and methane (CH4), potentially acting as a strong positive feedback to climate warming5,6,7. Thermokarst lake formation, a common abrupt thaw process, occurs due to the melting of excess ground ice in areas of permafrost degradation8. Such lakes are the most widespread feature due to abrupt permafrost thaw, and cover 7% of permafrost regions9. Due to their anaerobic environment, thermokarst lakes can be hot spots for CH4 emissions10,11,12, but in-situ measurements are sparse, especially from high-altitude permafrost regions, hampering our ability to assess the impact of abrupt thaw on the permafrost carbon cycle10,12,13. Therefore, improved understanding of CH4 emissions from alpine thermokarst lakes is crucial for predicting permafrost carbon-climate feedback in this climate-sensitive region.
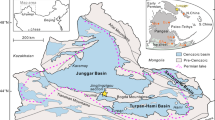
The Tibetan Plateau is the largest alpine permafrost region in the world (Fig. 1a), accounting for approximately 75% of the total alpine permafrost area in the Northern Hemisphere14. Similar to high-latitude permafrost regions, this region has experienced fast climate warming and extensive permafrost thaw3,15, which has triggered the widespread expansion of thermokarst lakes (Fig. 1b, c) and other types of abrupt permafrost thaw16. The number of thermokarst lakes in this permafrost region is estimated to be 161,300, with a total area of ∼2800 km2 17. Most of the lakes (~80%) are located in alpine grasslands which can be subdivided into alpine steppe, alpine meadow and swamp meadow17. Given that this permafrost region stores a large amount of SOC (15.3–46.2 Pg carbon in the top 3 m of soils; 1 Pg = 1015 g)18,19,20, permafrost thaw could facilitate the rapid microbial decomposition of organic matter, leading to substantial carbon emissions20,21. Additionally, the unique environmental conditions, characterized by lower air pressure and oxygen concentration due to the high elevation15,22, could be beneficial for CH4 production. Thermokarst lakes in this permafrost region are thus expected to behave as hot spots for CH4 emissions23,24. However, compared with the Arctic permafrost region, our understanding of CH4 emissions from alpine thermokarst lakes is limited. Specifically, relatively little is known about CH4 fluxes, the contribution of old carbon from thawing permafrost, methanogenic pathways (CO2 reduction versus acetate fermentation), and microbial characteristics (methanogenic functional genes and communities). These knowledge gaps prevent accurate prediction of the magnitude of carbon-climate feedback in this alpine permafrost region.
Our field sampling consists of the following three key steps. First, we choose 30 clusters of thermokarst lakes along a 1100 km transect on the Tibetan Plateau (a). Second, multiple locations within multiple lakes are selected at each cluster to eliminate spatial heterogeneity. In particular, four thermokarst lakes with different sizes are selected at each cluster (b). In each lake, 4 to 6 sampling locations are distributed from the shore to center (c), and at each location flux measurements are taken and averaged to estimate the CH4 and CO2 flux from the lake. Finally, each lake is sampled five times at monthly intervals during the ice-free period from mid-May to mid-October of 2021 to explore seasonal variation of CH4 or CO2 fluxes (d–h). In-situ CH4 and CO2 fluxes are determined using an opaque lightweight floating chamber equipped with a closed loop to a near-infrared laser CH4/CO2 analyzer (GLA231-GGA, ABB., Canada). In (a), the permafrost map of the Northern Hemisphere is obtained from the National Snow & Ice Data Center85. Spatial distribution of permafrost on the Tibetan Plateau is derived from Zou et al.86. The ellipses indicate the three representative permafrost regions in our study area, including the Madoi, Budongquan-Nagqu-Zadoi and Qilian sections. Photos are taken by G.Y.
In this context, we conducted a large-scale sampling campaign across 120 thermokarst lakes in 30 clusters (four lakes in each cluster: 4 lakes/cluster × 30 clusters) along a 1100 km transect on the Tibetan Plateau (Fig. 1a, b). Each lake was sampled five times at monthly intervals during the ice-free period from mid-May to mid-October of 2021 (Fig. 1d–h), with each campaign lasting ~25 days. We measured CH4 fluxes to the atmosphere using a portable opaque dynamic chamber (Supplementary Fig. 1). Our results show that the thermokarst lakes on the Tibetan Plateau are an important CH4 source (regional CH4 emission: 76.6 Gg (109 g) CH4 yr−1 with a mean flux of 13.4 ± 1.5 mmol m−2 d−1 during the ice-free period; hereafter, values are reported as mean ± standard error (SE) unless stated otherwise). Ebullition is the main pathway for CH4 release, contributing to 84% of CH4 emissions. Radio- and stable-carbon analyses show that old carbon is not the dominant source for CH4 production, with CH4 being derived mainly from the hydrogenotrophic pathway in most of the sampled lakes. The relative abundances of methanogenic genes correspond to the in-situ CH4 fluxes. These findings lay the groundwork for a comprehensive understanding of CH4 emissions in high-altitude thermokarst lakes.
Results and discussion
CH4 emissions across sampling clusters
Across the 30 sampled clusters, CH4 concentrations ranged from 107.1 to 159.4 nmol L−1 with a mean of 136 ± 30 nmol L−1 (n = 30; Supplementary Fig. 2a). CH4 was supersaturated relative to the local atmosphere in all the studied thermokarst lakes across the clusters with a mean value of 2921 ± 62% (ranging from 2393 to 3719%; n = 30). CH4 fluxes had high spatial variability within the 30 clusters, with values ranging from 0.1 to 39.2 mmol m−2 d−1 (Fig. 2). The lowest values occurred in thermokarst lakes located in alpine steppe (8.7 ± 3.0 mmol m−2 d−1) and the highest in alpine meadow (16.1 ± 1.7 mmol m−2 d−1). The CH4 fluxes also displayed temporal variability during the sampling period (Supplementary Fig. 3a). The maximum monthly mean CH4 flux was observed during the period of mid-June to mid-July (30.5 ± 4.9 mmol m−2 d−1), while the minimum occurred at the end of ice-free season (7.6 ± 1.5 mmol m−2 d−1), possibly due to the low temperature.
Bubble size is proportional to the value of the CH4 flux at each cluster, with a larger size representing a higher value. The background permafrost maps of the Northern Hemisphere and the Tibetan Plateau are derived from the National Snow & Ice Data Center85 and Zou et al.86, respectively. The inset shows the comparison of CH4 fluxes in thermokarst lakes located in the three grassland types. AS, AM and SM represent alpine steppe, alpine meadow and swamp meadow, respectively. Box plots present the 25th and the 75th quartile (interquartile range), and whiskers indicate the data range among thermokarst lakes located in AS (n = 5), AM (n = 13) and SM (n = 12), respectively. The notches are the medians with 95% confidence intervals. Observed values are shown as black dots. Significant differences are denoted by different letters (one-way ANOVAs with two-sided Tukey’s HSD multiple comparisons, p = 0.049).
The mean CH4 flux during the ice-free season was 13.4 ± 1.5 mmol m−2 d−1. This value is at the high end of the range reported from Arctic thermokarst water bodies regarded as hot spots for CH4 release13,25. During our field sampling campaign, we also measured CO2 emissions (see Supplementary Note 1 for detailed information). Our results showed that the contribution of CH4 to total carbon emissions (CH4 + CO2) from thermokarst lakes was 7.3% (Fig. 3a). If considered in terms of CO2-equivalent (CO2-e) emissions, mean CH4 flux during the ice-free season was estimated to be 136.8 CO2-e mmol m−2 d−1 (a 28-fold higher global warming potential relative to CO2 over 100 years)26, which was of the same order of magnitude as the CO2 emissions (170.4 mmol CO2 m−2 d−1; Supplementary Fig. 4) and approximately 44.6% of total CO2-e emissions (Fig. 3b). In conjunction with the total area of thermokarst lakes (~2800 km2)17, these findings demonstrate that alpine thermokarst lakes are hot spots of CH4 emission, and also highlight the importance of CH4 fluxes in the total carbon emissions from alpine thermokarst lakes. The high CH4 fluxes can be attributed to two characteristics of the Tibetan Plateau. First, atmospheric oxygen concentration across our study area is low due to the high elevation (between 3279 and 5014 m above sea level at our study sites), which can cause low dissolved oxygen concentration in all water bodies, including thermokarst lakes (4.3 ± 0.2 mg L−1; n = 30; Supplementary Table 1). Low dissolved oxygen concentration can stimulate CH4 production, and thus increase the CH4 flux27. Second, the thermokarst lakes across our study area are mostly shallow (depth range: ∼0.2-3.7 m; Supplementary Table 1)22. The shallow water column allows more rapid exchange with the atmosphere and less time for CH4 removal by microbial oxidation28, and is thus conducive to the release of CH4.
Panels (a, b) represent the density of carbon fluxes and CO2-equivalent emmissions, respectively. The lines indicate the fluxes from four thermokarst lakes at each cluster during the measurement period. The pie charts show the contribution of CH4 fluxes to total carbon emissions and CO2-equivalent emissions. Mean CH4 flux during the ice-free season and its CO2-equivalent emissions are shown outside parentheses, and the corresponding contribution to total carbon emissions and CO2-equivalent emissions are presented in parentheses, respectively.
CH4 diffusion and ebullition fluxes
To isolate the main pathways of CH4 release, we quantified both diffusion and ebullition fluxes. Ebullition occurred at all sampling clusters and exhibited a large spatiotemporal variability (Fig. 4a). Throughout the ice-free season, CH4 ebullition fluxes were highest from mid-June to mid-July with a mean value of 29.7 ± 4.7 mmol m−2 d−1 (n = 30; Supplementary Fig. 3a). During the ice-free period, mean CH4 diffusion across all the sampled clusters varied from 0.1 to 7.9 mmol m−2 d−1 while ebullition varied from 0.03 to 31.4 mmol m−2 d−1. The mean values were 2.1 ± 0.3 mmol m−2 d−1 for diffusion and 11.2 ± 1.5 mmol m−2 d−1 for ebullition (n = 30; Supplementary Fig. 3a). The maximum ebullition flux (200.8 mmol m−2 d−1) was recorded at the shore of one of the thermokarst lakes, where micro-bubbles were visible (Supplementary Movie 1). The contribution of ebullition to lake CH4 fluxes showed no significant difference among these three grassland types in which the thermokarst lakes are mainly distributed (p = 0.07; Supplementary Fig. 5a). Overall, alpine thermokarst lakes on the Tibetan Plateau were of high ebullition fluxes which constituted ~84% of the total CH4 fluxes (diffusion plus ebullition fluxes; Fig. 4a).
In (a), the corresponding values of light green and dark green lines represent the ebullition and diffusion CH4 fluxes across 30 clusters, respectively. Line shows the mean CH4 flux for the four thermokarst lakes at each cluster. In (b), line indicates the radiocarbon age of surface permafrost below the active layer at each sampling site (n = 24). In (c), the light blue and light orange lines are the apparent carbon fractionation factor (αC) values of 1.04 and 1.055. The αC values indicate the pathway of CH4 production, with αC > 1.055 suggesting that CH4 is mainly produced by CO2 reduction (hydrogenotrophic methanogenesis, HM), and αC < 1.055 suggesting that CH4 is produced increasingly by acetate fermentation (acetoclastic methanogenesis, AM). 14C and δ13C isotopic signatures were measured in the bubble gas from only 24 and 29 lakes respectively, due to the limited volume of gas samples that could be collected in the field.
The high contribution of ebullition to the total CH4 flux might be potentially explained by low atmospheric pressure on the Tibetan Plateau. Due to the high elevation, atmospheric pressure across the study area has a mean value of 60.9 kPa: roughly three-fifths of that at sea level (Supplementary Table 1). Two ways in which this low atmospheric pressure could result in a larger contribution of ebullition to the total CH4 flux. On the one hand, according to the Henry’s law, the lower atmospheric pressure causes lower CH4 solubility in the water29, which can be unfavorable for CH4 diffusion in the water column and thus impels CH4 to be transported from the lakebed to the atmosphere in the form of bubbles. On the other hand, atmospheric pressure can directly affect bubble formation. Bubbles containing CH4 occupy vertical tubes within the lake sediments30. The lower air pressure will benefit the vertical expansion of these bubbles and promote their escape from the lake sediment31, and is thus associated with greater ebullition of CH432. Consistently, the proportion of ebullition to total CH4 fluxes was negatively associated with air pressure (R2 = 0.42, p < 0.001; Supplementary Fig. 6a) but positively correlated with elevation across sampling clusters (R2 = 0.42, p < 0.001; Supplementary Fig. 6b). In addition, the shallowness of alpine thermokarst lakes on the Tibetan Plateau (Supplementary Table 1) could contribute to the high relative ebullition because shallow water creates less hydrostatic pressure22, increasing the formation and release of gas bubbles from lake sediments31,33.
Radiocarbon age of CH4 emissions
To estimate the contribution of old carbon to CH4 emissions, we collected ebullition gas samples with floating plastic bubble traps and determined the CH4 radiocarbon age. The results showed relatively young CH4 radiocarbon age, ranging from −360 to 3810 years before present (yrs BP; Fig. 4b). The mean CH4 radiocarbon age was only 325.8 yrs BP, and 46% of thermokarst lakes had modern (defined here as created after 1950) age of CH4 emissions (Supplementary Table 2). Only two lakes had higher CH4 radiocarbon ages than 1000 yrs BP, while radiocarbon ages were between modern and 1000 yrs BP in the remaining 22 samples. These observations indicated that old carbon was not the dominant source for CH4 production in most thermokarst lakes on the Tibetan Plateau. Potential explanations for the relatively low contribution of old carbon to CH4 fluxes observed in this study could be associated with the young permafrost carbon in this study region. It has been reported that permafrost on the Tibetan Plateau forms relatively recently compared to other permafrost regions34,35, which may lead to the situation that the frozen carbon is also relatively young. This deduction is supported by the measured average radiocarbon age of surface permafrost below the active layer at 24 sites across the Tibetan Plateau (6100 ± 880 yrs BP; n = 24; Supplementary Fig. 7; see Supplementary Note 2 for details of radiocarbon age measurements). Therefore, the relatively low contribution of old carbon to CH4 fluxes in alpine thermokarst lakes could be attributed to the young permafrost carbon in this study region. In addition, thermokarst lakes on the Tibetan Plateau are characterized by the small surface area22, and small lakes have a high perimeter to surface area ratio, which potentially increase terrestrial loading of organic matter from surrounding plants and surface soils. This terrestrial organic matter is dominated by modern carbon36, and can thus stimulate modern carbon emissions from thermokarst lakes37. The last but not the least, some of the studied lakes may have developed following thermokarst processes a long time ago or even not be thermokarst lakes. This might also be part of the explanation of the modern age of emitted CH4.
CH4 production pathway
To estimate the relative contribution of two major pathways of methanogenesis (CO2 reduction and acetate fermentation), we measured the δ13C of CH4 and CO2 in bubble samples to calculate the apparent carbon fractionation factor (αC); an indicator of the CH4 production pathway (see “Methods”; αC > 1.055 indicates CO2 reduction; αC < 1.04 indicates acetate fermentation)38. The δ13C–CH4 had a mean value of −72.5 ± 1.1‰ (n = 29), and the δ13C–CO2 ranged between −0.5‰ and −22.9‰ with a mean of −13.4‰ (Supplementary Table 2). Gas samples exhibited high αC values, ranging from 1.052 to 1.079 (1.064 ± 0.001, n = 29). Only four samples had αC values between 1.04 and 1.055, while the rest had αC values higher than 1.055 (Fig. 4c), indicating that CO2 reduction dominated CH4 production in alpine thermokarst lakes on the Tibetan Plateau. Furthermore, the αC values showed no significant difference among the three grassland types in which thermokast lakes are mainly distributed (p = 0.52; Supplementary Fig. 5b).
High αC values may be attributed to the alkaline and saline conditions across our study area (Supplementary Table 1). Specifically, high pH could stimulate the dissociation of acetic acid into its anion form (CH3COO−), which could then inhibit transmembrane diffusion and prevent the transportation of acetate39. Therefore, despite the accumulation of acetic acid, acetoclastic methanogenesis is likely to be less energetically favorable than hydrogenotrophic methanogenesis under alkaline conditions, leading to the high contribution of the CO2 reduction pathway to CH4 production40. Beside the alkaline conditions, saline environment-associated methanogenic substrates may be another potential driver for high αC values. In saline environment, methanol has been reported to be a methanogenic precursor and can serve as substrates for CH4 production41. Moreover, methanol-derived methanogenesis is usually accompanied by the highly depleted δ13C–CH4 values42. Consequently, methanol-dependent methanogenesis may also be responsible for the high αC values from alpine thermokarst lakes on the Tibetan Plateau due to their saline environment (ranging from 0.2 to 2.6 ppt; Supplementary Table 1).
Methanogenic microorganisms
To evaluate the potential differences in methanogenic microorganisms from thermokarst lakes in the three ecosystem types, we analyzed methanogenic functional genes and communities in surface sediment samples (0–15 cm) using metagenomic sequencing. Multiple functional genes of methanogens were more abundant in the thermokarst lakes located in alpine meadow and swamp meadow (Supplementary Fig. 8). This result suggested that there were higher potentials for CH4 production in thermokarst lakes located in these two grassland types, which matched with higher CH4 fluxes observed in the field at these locations (Fig. 2). The largest differences in relative abundance were observed for the mcr gene, with mean value being 4.1-fold and 3.2-fold higher in thermokarst lakes distributed in alpine meadow and swamp meadow than those located in alpine steppe, respectively (Supplementary Fig. 8). This result was confirmed by the validated mcrA gene predicted by contigs with length ≥ 1000 bp (Fig. 5a).
Panel (a) respresents the differences in the key functional gene of mcrA among thermokarst lakes located in alpine steppe (AS), alpine meadow (AM), and swamp meadow (SM). The relative abundance of mcrA gene was predicted by contigs with length ≥ 1000 bp. Box plots present the 25th and the 75th quartile (interquartile range), and whiskers indicate the data range among thermokarst lakes located in AS (n = 5), AM (n = 13) and SM (n = 12), respectively. The notches are the medians with 95% confidence intervals. Observed values are shown as black dots. Significant differences are denoted by different letters (one-way ANOVAs with two-sided Tukey’s HSD multiple comparisons, p = 0.038). Panel (b) shows methanogenic taxonomic infromation. The colors in the inner ring represent the different taxa. The triangles in the first ring indicate relative abundance with ≥ 5 per 10,000 (upper triangle) or <5 per 10,000 (lower triangle). The mean relative abundances for all samples are shown in the second ring and pillars, where color depth and height are proportional to the cubic root of relative abundance.
With regard to the sediment methanogenic community composition of the thermokarst lakes, there were five dominant methanogenic classes: Methanomicrobia, Methanobacteria, Thermoplasmata, Methanococci, and Methanopyri (Fig. 5b). The genus composition diagrams showed that Methanosarcina within Methanosarcinaceae order was the most abundant methanogenic genus, accounting for about 65% of all methanogens. It has been reported that Methanosarcina has a high volume-to-surface ratio with a large cell size and spherical form. Together with the formation of clusters, this leads to low levels of diffusion per unit cell mass43. Accordingly, Methanosarcina is relatively tolerant of adverse environmental conditions compared to other methanogens44. Moreover, the Methanosarcina genus contains cytochromes and methanophenazine (a functional menaquinone analog), which enable methanogens to conserve energy via membrane-bound electron transport chains so as to maintain high growth yields45. Overall, Methanosarcina can be competitive at low temperature, alkaline, and saline conditions (Supplementary Table 1), potentially contributing to high CH4 emission rates from alpine thermokarst lakes on the Tibetan Plateau.
Regional estimates
To upscale our lake-level measurements to regional efflux estimates, we conducted a Monte Carlo analysis to randomly sample thermokarst lake CH4 flux for each grassland type from a normal distribution around the mean. We then weighted the CH4 flux by the corresponding area of thermokarst lakes in each grassland type to calculate the regional flux. Total CH4 emissions were estimated to be 76.6 Gg CH4 yr−1. The contribution of CH4 to total carbon emissions was approximately 9.2% (Table 1), which was 1.4–8.4-fold greater than the mean contribution from lakes globally28,38,39,40. The 100-year global warming potential of CH4 was 2144.7 Gg CO2-e yr−1, which was approximately equivalent to lake CO2 emissions estimated in the same way (2084.7 Gg CO2 yr−1; Table 1). CH4 emissions from thermokarst lakes are often ignored when evaluating the regional CH4 balance across Tibetan alpine grasslands17. However, our results illustrated that CH4 emissions from thermokarst lakes could offset 15.3% of the CH4 uptake from alpine steppe and meadow which cover ~95% of alpine grasslands on the Tibetan Plateau46,47. Further, thermokarst lake CH4 emissions were equivalent to ~50–150% of the CH4 emissions from swamp meadow which occupies ~5% of Tibetan alpine grasslands18,47. The incorporation of CO2 fluxes gives an estimate of the overall carbon emissions (CH4 + CO2) in thermokarst lakes of 4.2 Tg (1012 g) CO2-e yr−1 (Table 1), which could offset 11.9% of net carbon sink in Tibetan alpine grasslands (37.1 Tg CO2-e yr−1)48. Taken together, these results demonstrate that any assessment of the carbon budget in this climate-sensitive region is incomplete without considering the significant carbon emissions from thermokarst lakes.
Although this study advances our understanding of CH4 release from thermokarst lakes on the Tibetan Plateau, it does have some limitations. First, carbon emissions during the ice-cover period were not considered in this study. To make a rough evaluation of ice-cover carbon emission, we applied the average percent contribution of ice-cover to annual carbon emissions from high-latitude lakes (CO2: 17%; CH4: 27%)49. These increased annual emissions estimate from alpine thermokarst lakes on the Tibetan Plateau to 427.0 Gg CO2 yr−1 and 28.3 Gg CH4 yr−1, respectively. Nevertheless, given the potential differences between high-latitude and high-altitude permafrost regions, such as climate, thermokarst lake depth and ice-cover duration34, the annual contribution of carbon emissions during the ice-cover period observed from high-latitude lakes may not be simply applied to thermokarst lakes in high-altitude permafrost region. More measurements during the ice-cover period are thus needed to further elucidate the role of thermokarst lakes in the regional carbon budget. Second, uncertainties exist in the area of the thermokarst lakes used in the regional carbon budget. Particularly, newly formed thermokarst lakes and thermokarst lakes located in desert and barren land were not considered, but they do have potential to contribute to CH4 emissions50. Meanwhile, theomarkast lake map used in this study suffers from uncertainties because it is based on low resolution and single satellite images and without consideration of ground-ice content17. It is thus essential to incorporate multi-satellite, higher spatial resolution remote sensing data (e.g. GF-2 and Planetscope) and ground-ice content to re-evaluate thermokarst lake area and its temporal dynamics in the future. Based on the updated lake distribution and the expansion rate, an improved estimation by considering thermokarst lakes distributed in other ecosystems and fresh thermokarst lakes is needed to obtain more accurate prediction of carbon release from alpine thermokarst lakes. Third, the development of thermokarst lake is not taken into account in this study. The studied lakes may cover the various thermokarst development stages or even non-thermokarst lakes, which can result in the uncertainty of the regional carbon emissions. Therefore, additional attention should be paid for the development of thermokarst lakes to further advance our understanding of CH4 emissions in alpine thermokarst lakes on the Tibetan Plateau.
In summary, this study offers an insight into the spatial patterns, sources and microbial characteristics of CH4 emissions from alpine thermokarst lakes on the Tibetan Plateau. Our results indicated that thermokarst lakes were an important but under-quantified CH4 source in the Tibetan alpine permafrost region. Considering that the expansion of thermokarst lake landscapes will accelerate under future climate warming, their potential contribution to the regional CH4 budget may increase substantially. Moreover, this study evaluated the landscape-level radiocarbon ages of thermokarst lake CH4 emissions in this alpine permafrost region. Our results illustrated that old carbon was not the dominant source for CH4 production from alpine thermokarst lakes on the Tibetan Plateau. This finding is in contrast to studies from the high-latitude permafrost region where old carbon contributes significantly to CH4 emissions upon permafrost thaw51. Hence, when modeling permafrost carbon-climate feedback across our study area, more efforts should be put into accurately predicting the effect of permafrost thaw on the spatial extent of thermokarst lakes, rather than on the rate of anaerobic decomposition of previously frozen soil. Furthermore, our results demonstrated that methanogenic functional genes corresponded with the in-situ CH4 fluxes, suggesting that methanogenesis might be the potential driver of CH4 emissions from alpine thermokarst lakes. Overall, multiple parameters observed in this study can function as benchmarks for better predicting permafrost carbon-climate feedback.
Methods
Study area
The Tibetan Plateau, the largest alpine permafrost region in the world, has a mean elevation of over 4000 m above sea level. Discontinuous and sporadic permafrost are widely distributed across the region52. Approximately 1.06 × 106 km2 of this region is underlain by permafrost, accounting for 40% of the overall plateau14. The permafrost mainly formed in the late Pleistocene during the Last Glaciation Maximum and the Neoglaciation period35. The current active layer thickness across the plateau is ~1.9 m, but this is deepening at a rate of ~1.3 cm yr−1 based on observations from 1981 to 201053,54. The climate is characterized as cold and dry14. Mean annual temperature ranges from −2.9 to 7.0 °C and mean annual precipitation varies from 129 to 590 mm, with approximately 90% of the precipitation falling during the growing season (late May to early October). The arid and semiarid climate has suppressed the development of ground-ice, leading to a relatively low ice content with a percentage by weight of 12.2% in this permafrost region34,55. The dominant vegetation types include alpine steppe, alpine meadow, and swamp meadow, with the corresponding dominant species being Stipa purpurea and Carex moorcroftii, Kobresia pygmaea and K. humilis, and K. tibetica, respectively. The plateau has experienced rapid climate warming, causing the formation and expansion of thermokarst lakes in various vegetation types (Supplementary Fig. 2c–e)56. The distribution of thermokarst lakes is dominated by lakes with small surface area (<10,000 m2) and shallow water which account for ~80% of the total number17. The mean ice-free duration of lakes across the study area is around 200 days57.
Flux and environmental measurements
In this study, we selected 30 clusters of thermokarst lakes for carbon flux measurements along a 1100 km transect on the Tibetan Plateau. The 30 clusters were evenly located in three representative permafrost regions (10 sites in the Madoi section on the eastern plateau, 15 sites in the Budongquan-Nagqu-Zadoi section, and 5 sites in the Qilian section on the northeastern plateau in the central part of the plateau; Fig. 1a). At each cluster, four thermokarst lakes were selected to cover different lake sizes. A total of 120 thermokarst lakes were thus sampled (30 clusters × 4 lakes/cluster). In each lake, 4–6 sampling locations were distributed from the shore to the center of the lake if the size allowed, and flux measurements were taken at each location and then averaged to estimate CH4 or CO2 fluxes from the respective lakes. This sampling at multiple locations within multiple lakes allowed us to consider the spatial variability of carbon fluxes in each cluster. Each thermokarst lake was sampled five times during the ice-free period from mid-May to mid-October of 2021 (once a month) to explore the seasonal variations of the CH4 and CO2 fluxes (120 lakes × 5 times). In-situ total CH4 and CO2 fluxes were determined between 9:00 a.m. and 18:00 p.m. using an opaque lightweight floating chamber (diameter: 26 cm, and height: 25 cm; Supplementary Fig. 1) equipped with a closed loop to a near-infrared laser CH4/CO2 analyzer (GLA231-GGA, ABB., Canada; Precision: <0.9 ppb CH4 (1 s) and 0.35 ppm CO2 (1 s); Measurement rates: 0.01 to 10 Hz.)33,58. Specifically, the floating chamber was flushed with ambient air for ~10 s before each measurement to ensure ambient CH4 and CO2 concentrations inside the chamber. The chamber was then pulled into its sampling location with a rope to avoid the need to enter the lake and potentially disturb sediment and gas release. CH4 and CO2 concentrations in the chamber were continuously recorded for 150 s at an interval of 1 sec after an equilibration period (20 s) to eliminate any disturbance of the surface boundary layer induced by chamber deployment. At each sampling location, measurements were made for 170 sec. This measurement time (170 s) was adopted to avoid CO2 accumulation in the floating chamber which could inhibit CO2 emission. The 20 s equilibration period was selected based on the time needed for CO2 concentration changes in the chamber to become linear. The 1 s interval was chosen to capture the change of gas concentration over time within the chamber in real time. Total CH4 and CO2 fluxes were calculated using the following Eq. 133,58:
where Ftotal is the total CH4 or CO2 flux (mmol m−2 d−1), nt and n0 represent the number of moles of CH4 or CO2 in the chamber at time 170 and 20 s after chamber deployment (mmol), respectively, A is the base area of the chamber (m2), t is the recording time during flux measurement (s).
Diffusion and ebullition CH4 fluxes were calculated using a two-layer model to estimate their relative contributions to the total CH4 release. Specifically, during flux measurements, 50 ml of surface water from a depth of 0–10 cm was collected with a 100 ml syringe (1:1 ratio of air-water). Subsequently, 50 ml of pure N2 was injected into the syringe to create 50 ml of headspace. The syringe was immediately shaken for 5 min to equilibrate the headspace in the field. The headspace sample was then injected into a vacuumed airtight vial and transported to the laboratory for analysis of CH4 and CO2 concentrations using a gas chromatograph (Agilent 7890 A, Agilent Technologies Inc., Santa Clara, Canada). Dissolved CH4 and CO2 concentrations were calculated using Henry’s Law adjusted for temperature and atmospheric pressure58. The degree of CH4 and CO2 saturation (S) was calculated by comparing the dissolved CH4 and CO2 concentration (CW) with the dissolved CH4 and CO2 concentration at equilibrium with the local atmosphere corrected for changes in solubility (Ceq) according to Eq. 2:33,58
Diffusion CH4 flux across the water surface into the chamber was estimated from Eq. 3:33,58
where Fd is the diffusion CH4 flux (mmol m−2 d−1), k is the CH4 transfer coefficient (m d−1), Cw is the dissolved CH4 concentration (mmol m−3), and Ceq is the CH4 concentration in water (mmol m−3) at equilibrium with the atmosphere in the field corrected for changes in solubility according to the Henry’s law29. The CH4 transfer coefficient (k) was estimated from Eq. 4:33,58
where kCO2 is the CO2 transfer coefficient, ScCH4 and ScCO2 are the Schmidt number of CH4 and CO2, respectively, n is 1/2 when wind speeds are >3.6 m s−1 or 2/3 if wind speeds are <3.6 m s−1 33. Given that CO2 ebullition is negligible due to its high solubility, CO2 is exclusively diffusive59. Due to this point, the CO2 transfer coefficient was calculated from Eq. 3.
Ebullition CH4 flux was determined from the difference between the measured total CH4 flux and the calculated diffusion CH4 flux. We would like to point out that, although the two-layer model is popularly used to evaluate CH4 diffusion flux from Arctic thermokarst lakes11,28 and alpine rivers33, uncertainties could be introduced due to the adopted theoretical gas transfer k coefficient60. Future studies should thus attempt to quantify CH4 ebullition and diffusion fluxes from alpine thermokarst lakes using other approaches (e.g. bubble traps61).
During flux measurement at each cluster (n = 30), we quantified wind speed and atmospheric temperature with a portable anemometer (Testo 480, Testo SE & Co. KGaA, Lenzkirch, Germany). The air pressure, water temperature, oxidation-reduction potentiality, pH and dissolved oxygen concentration were measured in each thermokarst lake (n = 120) with a portable multiparameter water quality instrument (ProSolo Digital Water Quality Meter, Yellow Springs Instrument, Brannum Lane, USA). Atmospheric CH4 and CO2 concentrations were also recorded with the CH4/CO2 analyzer (GLA231-GGA, ABB., Canada) as the steady values obtained when being flushed through with ambient air. All parameters were measured five times—once a month from mid-May to mid-October, 2021.
14C and δ13C isotopic analyses
To evaluate the CH4 radiocarbon age and the pathway of CH4 production, we quantified the 14C-CH4, δ13C-CH4, and δ13C-CO2 isotopic ratios. Due to the high cost of isotopic analyses, only one thermokarst lake at each cluster was randomly sampled for 14C and δ13C measurements. In addition, due to the limited gas samples, 14C and δ13C isotopic signatures were measured for only 24 and 29 lakes, respectively. Specifically, a combined bubble sample was collected using 4 submerged plastic traps (diameter 0.7 m) placed along the transect from the shore to the center of each of the selected lakes during mid-July to mid-August, 202162. Bubble gas from the traps was collected for about two weeks to enable sufficient volume to accumulate, and then divided into two parts. The first part was injected into 1 L pre-evacuated airtight gas-sampling aluminum bags (Dalian Delin Gas Packing Co., Ltd, China) for the determination of radiocarbon isotopic composition63. In particular, CO2 and H2O in the sample were removed using two traps which were filled with ethanol-liquid nitrogen and Alkali lime-Magnesium perchlorate, respectively. CH4 was then combusted with copper oxide to produce CO2 and H2O at 950 °C. Prior to this, the copper oxide was charged with oxygen at 600 °C overnight. Following combustion, water was removed through a trap filled with Alkali lime-Magnesium perchlorate, and the pure CO2 was then locked by a liquid nitrogen trap. Finally, the samples were quantified and catalytically reduced to graphite (containing ~1 mg C), and the 14C/12C isotopic ratio was measured by accelerator mass spectrometry (0.5MV 1.5SDH-1, NEC, USA) at Third Institute of Oceanography, Ministry of Natural Resources, Xiamen, China. The measured 14C values were corrected for mass-dependent fractionation by being normalized to a fixed δ13C value level64 and reported as conventional radiocarbon ages (years before present, yrs BP; where 0 yrs BP = AD 1950; Supplementary Table 2).
The second part of the gas sample was stored in 20 ml glass bottles for determining the stable-carbon isotopic composition of CH4 and CO238. Briefly, CH4 in the sample was purified through a trap filled with liquid nitrogen and then combusted to CO2 and H2O in an oxidation oven. After removing H2O, δ13C–CH4 was measured with an isotopic ratio mass spectrometry (IRMS 20-22, SerCon, Crewe, UK) at Institute of Botany, Chinese Academy of Sciences, Beijing, China. δ13C–CO2 measurements were made using a similar procedure, but without the purification and combustion. The apparent fractionation factor (αC) was calculated from δ13C of CH4 and CO2 (‰, relative to Vienna PDB) with Eq. 5:38
The αC value indicates the dominant pathway of methanogenesis; small αC values (1.04–1.055) are associated with acetate fermentation, while large αC values (1.055–1.09) are caused by CO2 reduction38.
Metagenomic sequencing, functional annotation and taxonomic analysis
To explore the potential role of the microbial community and functional genes in mediating CH4 emissions from thermokarst lakes, we collected lake sediment samples (0–15 cm) at equally spaced intervals along the transect from the shore to the center of each lake during mid-July to mid-August, 2021. The sediment samples were passed through a 2 mm sieve with visible roots being removed. Due to the high experimental cost, only one lake at each of the 30 clusters was sampled for metagenomic analysis. Lake sediment samples were immediately transported to the laboratory and stored at −20 °C until the metagenomic analysis. Total DNA was extracted from thawed sediment samples (0.4 g) using the DNeasy PowerSoil kit (Qiagen, Hilden, Germany) according to the manufacturers’ instructions. The ratios of spectrometry absorbance for the extracted DNA were between 1.7 and 1.9 at 260/280 nm and between 1.7 and 2.1 at 260/230 nm.
The metagenomic sequencing was carried out using the DNBSEQ-T7 platform (BGI, Shenzhen, China) to obtain 2 × 150 bp paired-end reads. Raw reads and adapters were first removed using SOAPnuke software (-n 0.001 -l 20 -q 0.4 --adaMR 0.25 --adaMis 3 --outQualSys 1) to generate trimmed reads consisting of approximately 15–18 Gb of sequencing data for each sample. Clean reads for each sample were assembled using Megahit (v.1.2.9)65, and contigs with length > 300 bp were retained. Then, open reading frames (ORF) were predicted by Prodigal (v.2.6.3)66, and dereplicated with 95% nucleotide identity using CD-HIT-EST (v.4.8.1)67. The translated proteins of non-redundant gene clusters were annotated by searching against the EggNOG (v.5.0) database68 using DIAMOND (v.2.0.9)69. The final annotation table was obtained from the KEGG database70 which is derived from EggNOG. To estimate the relative abundance of representative non-redundant genes in each sample, Salmon (v.1.5.1)71 was used to calculate the TPM (transcripts per million) of each predicted gene via mapping to clean paired reads of each sample. Taxanomic annotation was evaluated using Kraken2 (v.2.1.2)72 against the mini database (downloaded on Feb.−2022), and then visualized using GraPhlAn (v.0.9.7)73.
To further verify the annotations of genes involved in CH4 production, assembled contigs with length ≥1000 bp were used for identifying the methyl-CoM reductase alpha subunit (mcrA). Specifically, contigs with length ≥1000 bp were predicted using Prodigal (v.2.6.3) and then screened using HMM profiles (PF02249 and PF02745) derived from the Pfam database74 with hmmsearch (HMMER v.3.3.2)75. Putative mcrA genes were validated by phylogenetic tree with reference sequences derived from Annotree76 based on HMM profiles (PF02249 and PF02745 from Pfam) and Zhou et al.77. Putative mcrA proteins were aligned with reference sequences using MUSCLE (v.3.8.31)78 and built for a phylogenetic tree using FastTree (v.2.1.11)79 with the WAG + GAMMA models. Validated mcrA genes were mapped to clean paired reads of each sample by Salmon (v.1.5.1) to obtain counts, lengths and effective lengths. Counts per gene were normalized to reads per kilobase per million mapped reads (RPKM).
Statistical analyses
We conducted a series of statistical analyses to explore the basic characteristics of CH4 emissions from alpine thermokarst lakes. Specifically, one-way ANOVAs were carried out to evaluate the differences in total carbon emissions (CH4 + CO2), the contribution of ebullition to total CH4 fluxes, αC, the relative abundances of methanogenic functional genes and community from thermokarst lakes located in the three grassland types (alpine steppe, alpine meadow and swamp meadow). Tukey’s HSD difference test was used for multiple comparison at a significance level of α = 0.05. p value corrections were performed for multiple comparisons using the Benjamini–Hochberg correction factor80. Regression analyses were performed to examine the relationship of the ebullition proportion to total CH4 fluxes with elevation and air pressure. Before applying regression analyses, we conducted outlier analysis for the proportion of ebullition to total CH4 fluxes based on Boxplot Procedures81. Log transformation was conducted when the continuous variables and their residuals violated assumptions of normality.
We upscaled CH4 and CO2 fluxes from lake levels to the regional scale using Monte Carlo analysis that ran 1000 iterations for each of the grassland types (including alpine steppe, alpine meadow and swamp meadow) where thermokarst lakes are mainly located. Each iteration randomly resampled a CH4 or CO2 flux (for one of the three grassland types) based on a normal distribution surrounding the mean and standard deviation. Then, to generate the total thermokarst lake CH4 or CO2 flux per unit of time for each grassland type, we multiplied the randomly resampled CH4 or CO2 flux values by the area of thermokarst lakes for each grassland type which was determined by the distribution of thermokarst lakes on the Tibetan Plateau17 and the vegetation map was derived from China’s Vegetation Atlas82. Subsequently, we multiplied the total CH4 or CO2 flux per unit of time by the ice-free season duration (~200 d) to obtain the CH4 or CO2 emissions from each grassland type. Finally, we summed CH4 or CO2 emissions from each grassland type to estimate total CH4 or CO2 emissions. All statistical analyses were performed using R statistical software v3.6.2 (http://r-project.org)83.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings are available in the Figshare data repository (https://doi.org/10.6084/m9.figshare.22743968)84 and Supplementary Information. The nucleotide sequences generated by metagenome sequencing have been deposited in the NCBI database (ncbi.nlm.nih.gov/bioproject/?term=PRJNA942440).
References
Gruber, S. Derivation and analysis of a high-resolution estimate of global permafrost zonation. Cryosphere 6, 221–233 (2012).
Hugelius, G. et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 11, 6573–6593 (2014).
Biskaborn, B. K. et al. Permafrost is warming at a global scale. Nat. Commun. 10, 264 (2019).
Brown, J. & Romanovsky, V. E. Report from the international permafrost association: state of permafrost in the first decade of the 21st century. Permafr. Periglac. 19, 255–260 (2008).
Schuur, E. A. G. et al. Vulnerability of permafrost carbon to climate change: implications for the global carbon cycle. Bioscience 58, 701–714 (2008).
Schaefer, K. et al. The impact of the permafrost carbon feedback on global climate. Environ. Res. Lett. 9, 085003 (2014).
Schuur, E. A. G. et al. Climate change and the permafrost carbon feedback. Nature 520, 171–179 (2015).
Kokelj, S. V. & Jorgenson, M. T. Advances in thermokarst research. Permafr. Periglac. 24, 108–119 (2013).
Olefeldt, D. et al. Circumpolar distribution and carbon storage of thermokarst landscapes. Nat. Commun. 7, 13043 (2016).
Turetsky, M. R. et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 13, 138–143 (2020).
Serikova, S. et al. High carbon emissions from thermokarst lakes of Western Siberia. Nat. Commun. 10, 1552 (2019).
Walter Anthony, K. M. et al. 21st-century modeled permafrost carbon emissions accelerated by abrupt thaw beneath lakes. Nat. Commun. 9, 3262 (2018).
Wik, M. et al. Climate-sensitive northern lakes and ponds are critical components of methane release. Nat. Geosci. 9, 99–105 (2016).
Wang, B. & French, H. M. Permafrost on the Tibet Plateau, China. Quat. Sci. Rev. 14, 255–274 (1995).
Wang, B. et al. Tibetan Plateau warming and precipitation changes in East Asia. Geophys. Res. Lett. 35, L14702 (2008).
Li, L. et al. Thermokarst lake changes over the past 40 years in the Qinghai–Tibet Plateau, China. Front. Environ. Sci. 10, 1051086 (2022).
Wei, Z. et al. Sentinel-based inventory of thermokarst lakes and ponds across permafrost landscapes on the Qinghai-Tibet Plateau. Earth Space Sci. 8, e2021EA001950 (2021).
Ding, J. et al. The permafrost carbon inventory on the Tibetan Plateau: a new evaluation using deep sediment cores. Glob. Chang. Biol. 22, 2688–2701 (2016).
Wang, D. et al. A 1 km resolution soil organic carbon dataset for frozen ground in the Third Pole. Earth Syst. Sci. Data 13, 3453–3465 (2021).
Wang, T. et al. Permafrost thawing puts the frozen carbon at risk over the Tibetan Plateau. Sci. Adv. 6, eaaz3513 (2020).
Cheng, F. et al. Alpine permafrost could account for a quarter of thawed carbon based on Plio-Pleistocene paleoclimate analogue. Nat. Commun. 13, 1329 (2022).
Niu, F. et al. Characteristics of thermokarst lakes and their influence on permafrost in Qinghai–Tibet Plateau. Geomorphology 132, 222–233 (2011).
Mu, C. C. et al. High carbon emissions from thermokarst lakes and their determinants in the Tibet Plateau. Glob. Chang. Biol. 29, 2732–2745 (2023).
Wang, L. et al. High methane emissions from thermokarst lakes on the Tibetan Plateau are largely attributed to ebullition fluxes. Sci. Total Environ. 801, 149692 (2021).
Kuhn, M. A. et al. BAWLD-CH4: a comprehensive dataset of methane fluxes from boreal and arctic ecosystems. Earth Syst. Sci. Data 13, 5151–5189 (2021).
Masson-Delmotte, V. P. Z. et al. IPCC, 2021: Summary for policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, 2021).
Juutinen, S. et al. Methane dynamics in different boreal lake types. Biogeosciences 6, 209–223 (2009).
Holgerson, M. A. & Raymond, P. A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 9, 222–226 (2016).
Wiesenburg, D. A. & Guinasso, N. L. Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. J. Chem. Eng. Data 24, 356–360 (1979).
Martens, C. S. & Klump, J. V. Biogeochemical cycling in an organic-rich coastal marine basin—I. Methane sediment-water exchange processes. Geochim. Cosmochim. Acta 44, 471–490 (1980).
Mattson, M. D. & Likens, G. E. Air pressure and methane fluxes. Nature 347, 718–719 (1990).
Johnson, K. M. et al. Bottle-calibration static head space method for the determination of methane dissolved in seawater. Anal. Chem. 62, 2408–2412 (1990).
Zhang, L. et al. Significant methane ebullition from alpine permafrost rivers on the East Qinghai–Tibet Plateau. Nat. Geosci. 13, 349–354 (2020).
Wang, X. et al. Contrasting characteristics, changes, and linkages of permafrost between the Arctic and the Third Pole. Earth Sci. Rev. 230, 104042 (2022).
Jin, H. J. et al. Evolution of permafrost on the Qinghai-Xizang (Tibet) Plateau since the end of the late Pleistocene. J. Geophys. Res. 112, F02S09 (2007).
Dean, J. F. et al. Siberian Arctic inland waters emit mostly contemporary carbon. Nat. Commun. 11, 1627 (2020).
Elder, C. D. et al. Greenhouse gas emissions from diverse Arctic Alaskan lakes are dominated by young carbon. Nat. Clim. Chang. 8, 166–171 (2018).
Walter Anthony, K. M. et al. Methane production and bubble emissions from arctic lakes: Isotopic implications for source pathways and ages. J. Geophys. Res. 113, G00A08 (2008).
Welte, C. et al. Experimental evidence of an acetate transporter protein and characterization of acetate activation in aceticlastic methanogenesis of Methanosarcina mazei. FEMS Microbiol. Lett. 359, 147–153 (2014).
Wormald, R. M. et al. Hydrogenotrophic methanogenesis under alkaline conditions. Front. Microbiol. 11, 614227 (2020).
Conrad, R. Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: a mini review. Pedosphere 30, 25–39 (2020).
Conrad, R. Quantification of methanogenic pathways using stable carbon isotopic signatures: a review and a proposal. Org. Geochem. 36, 739–752 (2005).
Calli, B. et al. Methanogenic diversity in anaerobic bioreactors under extremely high ammonia levels. Enzym. Microb. Technol. 37, 448–455 (2005).
De Vrieze, J. et al. Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 112, 1–9 (2012).
Thauer, R. K. et al. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6, 579–591 (2008).
Wei, D. et al. Considerable methane uptake by alpine grasslands despite the cold climate: in situ measurements on the central Tibetan Plateau, 2008-2013. Glob. Change Biol. 21, 777–788 (2015).
Wei, D. et al. Revisiting the role of CH4 emissions from alpine wetlands on the Tibetan Plateau: evidence from two in situ measurements at 4758 and 4320m above sea level. J. Geophys. Res. Biogeosci. 120, 1741–1750 (2015).
Yan et al. The spatial and temporal dynamics of carbon budget in the alpine grasslands on the Qinghai-Tibetan Plateau using the Terrestrial Ecosystem Model. J. Clean. Prod. 107, 195–201 (2015).
Denfeld, B. A. et al. A synthesis of carbon dioxide and methane dynamics during the ice‐covered period of northern lakes. Limnol. Oceanogr. Lett. 3, 117–131 (2018).
Mu, C. et al. Dissolved organic carbon, CO2, and CH4 concentrations and their stable isotope ratios in thermokarst lakes on the Qinghai-Tibetan Plateau. J. Limnol. 75, 313–319 (2016).
Estop-Aragonés, C. et al. Assessing the potential for mobilization of old soil carbon after permafrost thaw: a synthesis of 14C measurements from the northern permafrost region. Glob. Biogeochem. Cycles 34, e2020GB006672 (2020).
Cheng, G. & Wu, T. Responses of permafrost to climate change and their environmental significance, Qinghai-Tibet Plateau. J. Geophys. Res. 112, F02S03 (2007).
Zhao, L. & Sheng, Y. Permafrost and its Changes on the Qinghai-Tibetan Plateau (Science Press, 2019).
Pang, Q. et al. Active layer thickness calculation over the Qinghai-Tibet Plateau. Cold Reg. Sci. Technol. 57, 23–28 (2009).
Zhao, L. et al. Estimates of the reserves of ground ice in permafrost regions on the Tibetan Plateau. J. Glaciol. Geocryol. 32, 1–9 (2010).
Gao, T. et al. Accelerating permafrost collapse on the eastern Tibetan Plateau. Environ. Res. Lett. 16, 054023 (2021).
Guo, L. et al. Uncertainty and variation of remotely sensed lake ice phenology across the Tibetan Plateau. Remote Sens. 10, 1534 (2018).
Bastviken, D. et al. Methane emissions from Pantanal, South America, during the low water season: toward more comprehensive sampling. Environ. Sci. Technol. 44, 5450–5455 (2010).
Sepulveda-Jauregui, A. et al. Methane and carbon dioxide emissions from 40 lakes along a north–south latitudinal transect in Alaska. Biogeosciences 12, 3197–3223 (2015).
Musenze, R. S. et al. Assessing the spatial and temporal variability of diffusive methane and nitrous oxide emissions from subtropical freshwater reservoirs. Environ. Sci. Technol. 48, 14499–14507 (2014).
Walter Anthony, K. M. et al. Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature 443, 71–75 (2006).
Bouchard, F. et al. Modern to millennium-old greenhouse gases emitted from ponds and lakes of the Eastern Canadian Arctic (Bylot Island, Nunavut). Biogeosciences 12, 7279–7298 (2015).
Walter Anthony, K. M. et al. Methane emissions proportional to permafrost carbon thawed in Arctic lakes since the 1950s. Nat. Geosci. 9, 679–682 (2016).
Garnett, M. H. et al. Radiocarbon analysis of methane emitted from the surface of a raised peat bog. Soil Biol. Biochem. 50, 158–163 (2012).
Li, D. et al. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 102, 3–11 (2016).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010).
Fu, L. et al. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012).
Huerta-Cepas, J. et al. EggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314 (2018).
Buchfink, B., Reuter, K. & Drost, H. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 18, 366–368 (2021).
Kanehisa, M. et al. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462 (2016).
Patro, R. et al. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Wood, D. E. et al. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257 (2019).
Asnicar, F. et al. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ 3, e1029 (2015).
Finn, R. D. Pfam: the Protein Families Database (Encyclopedia of Genetics, Genomics, Proteomics and Bioinformatics, 2005).
Jaina, M. et al. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 41, e121 (2013).
Mendler, K. et al. AnnoTree: visualization and exploration of a functionally annotated microbial tree of life. Nucleic Acids Res. 47, 4442–4448 (2019).
Zhou, Z. et al. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species. Nature 601, 257–262 (2022).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Sim, C. H. et al. Outlier labeling with boxplot procedures. J. Am. Stat. Assoc. 100, 642–652 (2005).
Editorial Board of Vegetation Map of China. Vegetation Atlas of China (Science Press, 2001).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).
Yang, G. B. et al. Characteristics of methane emissions from alpine thermokarst lakes on the Tibetan Plateau. Dataset. Figshare. https://doi.org/10.6084/m9.figshare.22743968 (2023).
Brown, J. et al. Circum-arctic map of permafrost and ground-ice conditions, version 2. [Data Set]. Boulder, Colorado USA. NASA National Snow and Ice Data Center Distributed Active Archive Center (accessed 5 May 2023). https://doi.org/10.7265/skbgkf16 (2002).
Zou, D. et al. A new map of permafrost distribution on the Tibetan Plateau. Cryosphere 11, 2527–2542 (2017).
Acknowledgements
We thank Dr. Liwei Zhang (College of Urban and Environmental Sciences, Peking University) for valuable advice on flux measurement and calculation, Dr. Pengfei Liu (Center for the Pan-Third Pole Environment, Lanzhou University) for helpful suggestions regarding bioinformatic analyses and data interpretations, and Dr. Katey Walter Anthony (Water and Environmental Research Centre, University of Alaska Fairbanks) for constructive comments on an early version of the manuscript. Permissions to work and collect gas and sediment samples across the study area were granted by the Three-River-Source National Park Management Bureau. This work was supported by the National Natural Science Foundation of China (31988102 and 31825006, Y.Y., and 32201359, G.Y.), National Key Research and Development Program of China (2022YFF0801903, Y.Y.), and China National Postdoctoral Program for Innovative Talents (BX20200363, G.Y.).
Author information
Authors and Affiliations
Contributions
Y.Y. and G.Y. designed this research. G.Y. and Z.Z. performed field samplings. G.Y., Z.Z., L.K. and S.Q. performed laboratory experiments. G.Y., Y.S., L.K. and Y.P. analysed data. G.Y., B.A., D.O., C.K. and Y.Y. wrote the manuscript with input from other co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Blaize Denfeld and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, G., Zheng, Z., Abbott, B.W. et al. Characteristics of methane emissions from alpine thermokarst lakes on the Tibetan Plateau. Nat Commun 14, 3121 (2023). https://doi.org/10.1038/s41467-023-38907-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-38907-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.