Abstract
Otosclerosis is one of the most common causes of conductive hearing loss, affecting 0.3% of the population. It typically presents in adulthood and half of the patients have a positive family history. The pathophysiology of otosclerosis is poorly understood. A previous genome-wide association study (GWAS) identified a single association locus in an intronic region of RELN. Here, we report a meta-analysis of GWAS studies of otosclerosis in three population-based biobanks comprising 3504 cases and 861,198 controls. We identify 23 novel risk loci (p < 5 × 10−8) and report an association in RELN and three previously reported candidate gene or linkage regions (TGFB1, MEPE, and OTSC7). We demonstrate developmental stage-dependent immunostaining patterns of MEPE and RUNX2 in mouse otic capsules. In most association loci, the nearest protein-coding genes are implicated in bone remodelling, mineralization or severe skeletal disorders. We highlight multiple genes involved in transforming growth factor beta signalling for follow-up studies.
Similar content being viewed by others
Introduction
Otosclerosis is an exclusively human disorder characterized by pathologic remodeling of the bone encasing the inner ear, called the otic capsule, and is one of the most common causes of conductive hearing loss1,2. Hearing begins when sound-induced vibrations of the tympanic membrane and ossicles within the middle ear are transmitted via stapes footplate within the oval window to sensory cells of the inner ear. In classic otosclerosis, the conduction of sound through the ossicular chain is impeded due to fixation of the stapes footplate by pathologic bone remodeling, leading to conductive hearing loss. When bone remodeling progresses to involve the cochlear endosteum, otosclerosis can cause additional sensorineural hearing loss in 20–30% of patients, which reflects damage to the delicate intracochlear cells. Although genetic, viral, immunologic and vascular factors have been implicated, the pathogenesis of otosclerosis remains poorly understood. Therapeutic options include hearing aids which provide amplification, stapedotomy surgery which replaces the fixed stapes bone with a mobile prosthesis, and cochlear implant surgery which facilitates direct electrical stimulation of the auditory nerve in most advanced cases of otosclerosis. However, robust pharmacological or genetic therapies for otosclerosis do not yet exist.
Clinical otosclerosis has an estimated prevalence of 0.30–0.38% in populations of European descent3. Histologic otosclerosis without clinical symptoms is more frequent, with bony overgrowth observed in as many as 2.5% of temporal bone autopsy specimens4. Symptomatic otosclerosis most frequently occurs in working-age individuals between the second and fifth decades5,6. Initial manifestation is often limited to one ear, but eventual bilateral disease is observed in 70–80% of cases5,6.
Otosclerosis is highly familial, with a positive family history reported for 50–60% of cases7,8. Based on segregation patterns, early studies classified otosclerosis as an autosomal dominant disease with reduced penetrance8,9,10. However, efforts to identify causative genes have produced inconsistent results, with insufficient evidence for most candidate genes11,12. Individual targeted next-generation sequencing studies have identified potential susceptibility variants in SERPINF1, MEPE, ACAN and FOXL1, although a follow-up study of SERPINF1 did not replicate the signal12,13,14,15,16. A genome-wide association study (GWAS) including a total of 1149 otosclerosis patients identified several intronic variants within the gene encoding Reelin (RELN) that were associated with otosclerosis in European populations17. Although expressed in the inner ear, the molecular mechanism by which Reelin affects otosclerosis susceptibility is unknown.
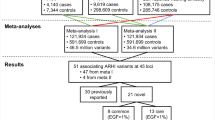
Here, we report to our knowledge the largest GWAS of otosclerosis including a total of 3504 cases and 861,198 controls from three population-based sample collections: the Finnish FinnGen study, the Estonian Biobank (EstBB), and the UK Biobank (UKBB). We identify 23 novel GWAS loci and report associations in RELN, in two previously reported candidate genes (TGFB1 and MEPE) and in the OTSC7 linkage region17,18,19,20,21,22. Several discovered loci harbor genes involved in the regulation of osteoblast or osteoclast function or biomineralization. Genomic risk loci for otosclerosis also overlap with risk loci for rare skeletal disorders.
Results
Identification of otosclerosis cases based on electronic health records
Based on International Classification of Diseases (ICD) diagnosis codes (versions 8, 9, and 10), we identified a total of 3504 otosclerosis cases in the three biobanks, including 1563 cases in FinnGen, 985 in EstBB and 956 in UKBB, for cohort prevalences of 0.62%, 0.50%, and 0.23%, respectively (Table 1). A total of 861,198 individuals had no ICD-based diagnosis of otosclerosis and were assigned control status. A proportion of otosclerosis patients undergo stapes procedures, which reflect disease severity and the validity of the ICD-based diagnoses. Stapes procedures were registered for 616 (39.4%) otosclerosis cases in FinnGen and for 218 (22.1%) otosclerosis cases in EstBB. In UKBB, reflecting the ascertainment of a large proportion of cases based on procedural history, stapes procedures were registered for 73.2% of cases. The gender and age distribution of controls and cases in each cohort is presented in Supplementary Data 1.
Genomic loci associated with otosclerosis in each cohort
In case-control GWASs within the individual cohorts, we observed eleven loci associated with otosclerosis at genome-wide significance (p < 5×10−8) in FinnGen, two in UKBB and one in EstBB (Supplementary Fig. 1; Supplementary Data 2-4). One locus on chromosome 11 was associated in both FinnGen and UKBB at genome-wide significance, tagged by the lead variants rs11601767 (closest protein coding gene: LTBP3) and rs17676161 (SCYL1), respectively. The only genome-wide significant variant in EstBB was rare (MAF 0.001%) and was not included in FinnGen or UKBB.
Single-nucleotide polymorphism (SNP) based heritability, calculated from the summary statistics, was higher in FinnGen (h2 range on the liability scale = 0.28–0.69) than in EstBB (range = −0.09–0.23) or UKBB (range = 0.10–0.21) (Supplementary Data 5 and Supplementary Fig. 2). Attenuation ratio statistics calculated using cross-trait LD Score Regression (LDSC) were 0.36 (SE 0.11) for FinnGen, 2.36 (1.14) for EstBB and <0 for UKBB, respectively (Supplementary Data 6)23,24. We calculated pairwise genetic correlation rg with LDSC using summary statistics from the different cohorts. Rg between FinnGen and UKBB was 0.70, indicating shared genetic etiology between the phenotype definitions23. The calculation of rg with LDSC was not feasible for EstBB due to a negative heritability estimate with LDSC (observed scale h2 = −0.002 [S.E. 0.0023]), likely reflecting modest polygenic signal in the cohort. However, as the effect estimates for lead variants were largely concordant between the three cohorts (Supplementary Data 2-5), we proceeded with a fixed-effects meta-analysis.
Twenty seven significant loci in the meta-analysis of summary statistics
In the genome-wide meta-analysis of all three cohorts (including a total of 3504 cases and 861,198 controls) we identified 1452 variants associated with otosclerosis at the genome-wide significance level (p < 5×10−8). The genomic inflation factor (λGC) calculated from the meta-analysis p-values was 1.046, while the univariate LD Score regression intercept was closer to 1.0 at 1.02 (S.E. 0.01), indicating that part of the elevation in λGC reflected true additive polygenic effects and not spurious associations from population stratification or cryptic relatedness24. We used several methods to estimate the heritability of otosclerosis based on genome-wide meta-analysis summary statistics. The SNP-based heritability of otosclerosis was 0.15 (SD = 0.03) when estimated with LDSC, 0.23 (SD = 0.01) when estimated with LDAK-THIN and 0.27 (0.03) when estimated with BLD-LDAK (Supplementary Data 5).
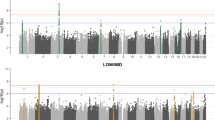
The significant variants in the meta-analysis clustered in a total of 27 loci (regions at least 1.5MB apart) (Fig. 1; Table 2; Supplementary Data 7; Supplementary Fig. 3), including the previously reported RELN locus and 26 loci not previously reported in a GWAS study of otosclerosis17. All but one of the lead variants in the 27 loci were common with a cross-cohort effect allele frequency (EAF) of at least 11% (Table 2). We also report suggestive-level loci (p < 1×10−6) not reaching genome-wide significance in Supplementary Data 8.
GWAS for each individual study cohort was performed using a generalized mixed model with the saddlepoint approximation using SAIGE v0.20, using a kinship matrix as a random effect and covariates as fixed effects. Results are presented for a fixed-effect meta-analysis of effect estimates from the three cohorts, including a total of 3504 cases and 861,198 controls. In the Manhattan plot, chromosomal positions are indicated on the x-axis and -log10(p-value) is presented on the y-axis for each variant. Twenty-seven loci reached genome-wide significance (two-sided p-value < 5×10−8 to account for multiple comparisons, marked by the dashed line). The included variants were present in at least two cohorts with a cross-cohort minor allele frequency > 0.1% and imputation INFO score ≥ 0.7. The loci are annotated by the names of the coding genes nearest to the lead variants. The exact p-values corresponding to lead variants in each locus are presented in Table 2. Blue and gray colours are used to visually separate chromosomes.
Two of the GWAS meta-analysis lead variants (Near MEPE and SUPT3H) are driven by their association in FinnGen. The variant rs181831514 near MEPE is strongly enriched in Finland, with very low frequency in EstBB and UKBB. The lead variant rs13192457 in the association locus overlapping SUPT3H was not replicated at nominal significance in UKBB (p > 0.05) and was not included in EstBB (Supplementary Data 7). However, the second-most significant variant in the SUPT3H locus (rs12204678, 6:45153962:C > T, meta-analysis p = 8.3 × 10−11, MAF = 41%, OR = 0.85 [0.81–0.9]) was included in EstBB and was associated with otosclerosis at nominal significance (p = 0.029, OR = 0.90 [0.86-0.95]).
In two of the 27 genome-wide significant loci, the nearest genes (MEPE and TGFB1) have been implicated in candidate gene studies. One locus (denoted by the lead variant rs4464751 in an intron of CD109) is a previously identified linkage locus (OTSC7, 6q13–16.1)25. To our knowledge, the remaining 23 loci have not been characterized in association with otosclerosis18,19,20,21,22.
Characterization of the potential susceptibility loci for otosclerosis
The lead variant in the chromosome 3 locus, rs4917 T > C, is a missense variant in the exon 6 of AHSG (EAF = 64%, OR = 0.84 [0.80–0.88], p = 3.4 × 10−12). The variant results in a methionine to threonine conversion and is predicted to be tolerated/benign by the SIFT and PolyPhen algorithms with a scaled CADD score of 9.9.
In the chromosome 4 locus near the MEPE gene, the rare frameshift variant rs753138805 (EAF 0.3% in controls and 1.3% in cases) was the lead variant in the GWAS in FinnGen with an odds ratio of 21.5 (95% CI 9.6-48.4) (p = 9.8 × 10−14). The variant is 2.4-fold enriched in Finnish compared with non-Finnish Europeans based on sequence data in the Genome Aggregation Database26. It was not included in the meta-analysis due to poor imputation quality in EstBB (Imputation information score 0.28) and non-inclusion in UKBB genotype data.
Among all 1,452 variants which reached genome-wide significance in the meta-analysis, thirteen missense variants were significantly associated with otosclerosis (Supplementary Data 9). One, rs140145986 in RIN1 (EAF = 8.6%, OR = 1.3 [1.19-1.41], p = 4.1 × 10−10), was classified as deleterious and probably damaging by the SIFT and PolyPhen algorithms respectively, with a scaled CADD score of 23.1 (Supplementary Fig. 3).
Next, based on linkage disequilibrium in the FinnGen cohort, we examined 1142 variants (including those with AF < 0.001) in high LD (r2 > 0.6) with the lead variants from the meta-analysis. We observed one additional protein-altering variant, rs11526468 in SEMA4D (EAF = 30%, OR = 0.91 [0.87-0.96], p = 0.00039; Supplementary Data 10; Supplementary Fig. 3). The variant, resulting in an alanine to threonine conversion, is predicted to deleterious and probably damaging by the SIFT and PolyPhen algorithms, respectively, with a scaled CADD score of 24.3.
Finally, the lead variants were intronic for protein-coding genes in fourteen loci (Table 2). The functional assessment of such variation is more challenging. In the locus on chromosome 7, the strongest association was observed for variants in the second and third introns of RELN concordant with previous GWAS17,18. In a locus on chromosome 19, the association signal overlapped with several genes including TGFB1, AXL, HNRNPUL1 and CCDC97, with the only genome-wide significant association observed for the intronic TGFB1 variant rs8105161 (EAF = 16%, OR = 0.83 [0.78-0.89], p = 2.7 × 10−8). On chromosome 6, the association signal only overlapped the CD109 gene within the wider OTSC7 linkage region. In two of the significant loci, the associated variants were tightly clustered in the region of antisense genes: COL4A2-AS2 and an intronic region of COL4A2 on chromosome 13, and STX-17-AS1 on chromosome 9. On chromosome 6, an apparent association haplotype block spanned the entire SUPT3H gene and the first exon and first intron of the RUNX2 gene; the lead variant rs13192457 (EAF = 44%, OR = 1.21 [1.14–1.28], p = 4.8 × 10−11) is located in an intronic region of SUPT3H.
Fine-mapping of the association loci
To identify the most likely causal variants in the otosclerosis association loci, we performed fine-mapping analyses in FinnGen using the SuSie software v.1.027. We analyzed all loci that had suggestive evidence of association in FinnGen (p < 1 × 10−6). The number of variants in good-quality credible sets ranged from three (for the chromosome 4 locus near MEPE) to 156. (Supplementary Data 11 and 12). In the chromosome 4 locus, the MEPE frameshift variant rs753138805 was the most likely causal variant (posterior inclusion probability [PIP] = 40%) (Supplementary Data 12). No other coding variants identified in previous analyses (e.g. rs140145986 in RIN1 or rs11526468 in SEMA4D) were highlighted by fine-mapping. In the chromosome 16 locus near PTX4, all credible set variants were located upstream of or within the first intron of CLCN7, a gene associated with osteopetrosis, but the significance of this finding is uncertain28.
Phenome-wide association studies of the 27 otosclerosis lead variants
To assess pleiotropic effects of genetic risk factors to otosclerosis, we examined the phenome-wide associations of the 27 lead variants from the meta-analysis with a total of 4756 traits registered in the GWAS Atlas. Lead variants were frequently associated with several traits at the bonferroni-corrected threshold of p < 1.05×10−5: twelve lead variants were associated with heel bone mineral density (BMD), six with sitting height, four with standing height, three with hip osteoarthritis, three with FEV1/FVC ratio, three with trunk impedance measures (fat-free mass, trunk predicted mass and impedance of arm), and three with body mass index (Supplementary Data 13 and 14). We observed significant discordance between the direction of effect for otosclerosis and heel bone mineral density when evaluating all variants reaching nominal significance in GWAS Atlas (2 directionally concordant co-associations and 15 directionally discordant co-associations, p-value = 0.0023; Supplementary Data 15).
As we found no reported associations in the GWAS Atlas for the rare MEPE rs753138805 frameshift variant, we performed a separate phenome-wide association study for the variant in FinnGen. Among 2,861 total traits, in addition to otosclerosis and hearing loss, rs753138805 was significantly associated with fractures of the lower leg (beta = 0.53, p = 1.5 × 10−5, Supplementary Data 16).
We also examined the correlation of effect estimates for otosclerosis and recurring traits from the PheWAS for all meta-analysis lead variants in the FinnGen cohort. Bone mineral density was not available, but we could evaluate effect estimates for osteoporosis and risk of fractures. None of the associations remained statistically significant after correction for multiple comparison (Supplementary Fig. 4).
Analyses of shared heritability
Following frequently observed phenotypes in the phenome-wide association analysis of the lead variants, we tested the genetic correlations of otosclerosis with 54 bone-related, anthropometric and lung traits in LDHub23,24,29. None of the traits were significantly genetically correlated with otosclerosis after multiple correction (p > 0.00093 for all traits) (Supplementary Data 17).
Rare disease associations in GWAS Catalog and ClinVar
We queried the GWAS Catalog and ClinVar database for rare disease associations for all 1452 variants associated with otosclerosis at genome-wide significance (p < 5 × 10−8). In the chromosome 8 locus overlapping EIF3H, rs13279799 (OR for otosclerosis = 0.77 [0.71–0.83]; p = 2.8×10−11) has previously been associated with ossification of the longitudinal ligament of spine, a rare form of heterotopic ossification (Supplementary Data 18)30. Both rs13279799 and the fine-mapped credible set were located downstream of EIF3H. None of the 1452 variants which were associated with otosclerosis at genome-wide significance, nor any variants associated with otosclerosis at nominal significance (p < 0.05) within the association loci, were reported as pathogenic for diseases registered in ClinVar.
Gene-based and gene set analysis
In a gene-based analysis using MAGMA, a total of 53 genes were significantly associated with otosclerosis (p < 2.65 × 10−6) (Supplementary Data 19). The gene-based associations were significantly enriched (p < 4.9 × 10−6) for two gene sets based on gene ontology terms: COLLAGEN TYPE IV TRIMER (p = 1.9 × 10−6) and BASEMENT MEMBRANE COLLAGEN TRIMER (p = 1.2 × 10−6).
In a GO Biological Process (BP) enrichment analysis of the 27 protein-coding genes nearest to the lead variants, we found enrichment of nine main BP categories and a total of 16 BPs (false discovery rate corrected p-value < 0.05) (Supplementary Data 20). All enriched categories were relevant for bone metabolism, biomineralization, skeletal system development, positive regulation of cell development, or TGFβ receptor signalling.
Replication of previous otosclerosis candidate genes and linkage loci
As two of the genome-wide significant loci from the meta-analysis overlapped with candidate genes (TGFB1 and MEPE) and one association locus overlapped with the OTSC7 linkage locus, we also examined the association signals near other candidate genes and linkage loci (Supplementary Data 21; Supplementary Fig. 5). With the exception of the RELN variant rs3914132 identified in the previous GWAS study, we observed no significant associations for previously reported susceptibility variants including the recently reported FOXL1 deletion (AF = 0.002, OR = 1.11 [0.62-2], p = 0.72)16,31,32,33. None of the previously reported susceptibility variants in SERPINF1 or ACAN were included in the current meta-analysis, but we observed no suggestive association signals (p < 1 × 10−6) in a 3MB window surrounding SERPINF1 or ACAN12,13. Finally, we assessed all previously reported linkage loci, but found no suggestive associations with the exception of the genome-wide significant association within OTSC7.
Colocalization
As the risk loci could alter otosclerosis susceptibility via gene expression, we evaluated colocalization between the otosclerosis meta-analysis and expression datasets available in the eQTL catalogue. As no data on bone expression was available, we focused on genes with altered expression across at least two tissue types with a causal posterior probability of ≥ 0.1%. Fourteen such genes were identified (Supplementary Data 22 and 23). Otosclerosis colocalized genetically with the expression of BANF1, MARK3 and SUPT3H in the highest number of tissues (14, 8 and 6, respectively), while the highest causal posterior probability was observed for TELO2 (9.5%), ZNRD2 (6.3%), EHBP1L1 (6.0%).
Validation of otosclerosis gene candidates in the mammalian cochlea
To investigate the pattern of expression of candidate genes, we performed immunostaining on neonatal and adult mouse cochleae for the proteins encoded by RUNX2 and MEPE genes. We found strong nuclear expression of RUNX2 in osteoblasts within the otic capsule only on postnatal day 2 (P2)-old mice (Fig. 2b) and the absence of RUNX2 immunostaining in the adult inner ear (Fig. 2g). Immunostaining for MEPE revealed dynamic changes through postnatal development: the pattern was more diffuse at P2 (Fig. 2c), then limited to maturing osteocytes within the otic capsule of postnatal day 6 (P6)-old mice (Fig. 2d), and persisted in mature osteocytes of postnatal day 12 (P12)-old mice (Fig. 2a, e) and 3-month-old mice (Fig. 2f).
a Cross-section through a P12 mouse cochlea stained with anti-MEPE-antibody (brown) and counterstained with hematoxylin (blue). b Immunofluorescent staining for RUNX2 (green) was observed in the nuclei (blue) of P2 osteoblasts. g Lack of RUNX2 expression in the adult otic capsule. c – f Immunofluorescence for MEPE (green) developed from more diffuse (c white arrowhead) to more clearly cellularly localized (f white arrows) with age. SL Spiral ligament. Representative images of N = 3 animals per age group. Scale bars: a: 500 µm, b–g 50 µm.
Discussion
Although otosclerosis is highly heritable its genetic background is still poorly understood. Previous studies have reported one GWAS locus (RELN), while results for candidate genes have been inconclusive. Here, we identify 27 loci associated with otosclerosis, of which 23 are novel. We also identify four previously reported loci: the RELN locus, the loci of two candidate genes (TGFB1 and MEPE) and the OTSC7 linkage locus. These results offer further insights to disease pathophysiology, especially when interpreted in light of the dynamic communication between the bony otic capsule and the intracochlear soft tissues encased by this bone. Specifically, unlike most other bones in the body that continuously remodel throughout life, the normal adult otic capsule remodels minimally if at all34. This lack of remodeling is achieved, at least in part, through molecules secreted by intracochlear soft tissues, such as osteoprotegerin (OPG), which diffuse into the surrounding otic capsule35,36.
The novel loci we identified are highly relevant because of known histopathologic and clinical features of otosclerosis. Histologically, otosclerosis is characterized by pathologic bone remodelling that starts at sites of predilection, which are globuli interossei, embryonic rests of cells from the original cartilaginous framework since the otic capsule is formed through endochondral ossification. Clinically, otosclerosis is similar to other diseases that cause stapes fixation, such as osteogenesis imperfecta, multiple-synostoses syndrome and congenital X-linked deafness with stapes gusher; the genes identified in our study are related to the genes known to cause stapes fixation in other diseases.
We confirm a polygenic basis to otosclerosis. Early genetic studies of otosclerosis assumed a dominant inheritance, but modest success in mapping otosclerosis to one or a few high-impact genes, even in family based linkage studies, has suggested a complex nature. At an individual level, genetic disease susceptibility could reflect the contribution of multiple low-impact or few high-impact variants. Many etiologies to otosclerosis have been proposed, including differences in TGFβ, parathyroid hormone or angiotensin II signalling, alterations in collagen type I, inflammation, viral infection, and autoimmunity37. The association loci include several genes involved in the regulation of bone structure; we validated two of these genes, RUNX2 and MEPE, via immunostaining of inner ear sections for the corresponding proteins. Follow-up functional studies are expected to reveal additional molecular insights.
In a considerable number of the loci, either the nearest protein-coding gene or a gene whose expression colocalizes genetically with otosclerosis is associated with severe skeletal disorders (Supplementary Table 1). Disordered or heterotopic bone growth is a common feature in diaphyseal dysplasia (as caused by mutations in TGFB1), osteopetrosis subtypes (as caused by mutations in CLCN7 and TNFSF11 which codes for RANKL) and ossification of the posterior longitudinal ligament of spine (previously associated with rs13279799 for which we report an association with otosclerosis). The cochlear RANKL-RANK-OPG axis is critical for regulation of not only bone remodeling within the otic capsule, but also neuronal survival and outgrowth35,36,38,39. Compared with mono- or oligogenic severe skeletal dysplasias, otosclerosis presents later in adulthood and represents a more limited and common phenotype. Genetic susceptibility to otosclerosis in the population may reflect more moderate and aggregate polygenic disruption of bone growth and regulation.
The effects on bone growth and remodeling in otosclerosis may be mediated by several pathways. The known biological functions of many genes within the otosclerosis association loci include regulation of osteoblasts – the cells responsible for bone formation – and osteoclasts – responsible for the breakdown of bone, either through effects on cell differentiation or function (Supplementary Table 1). Some genes (e.g. AHSG, IRX5, MEPE, FAM20A and SMAD3) are also directly involved in biomineralization, and AHSG and the candidate gene MEPE have been reported to have dual roles in the regulation of mineralization and osteoclast, osteoblast or osteocyte cell lineages. Our localization of MEPE protein within the maturing and adult otic capsule validates our GWAS finding.
Recently, Schrauwen and colleagues proposed a model of otosclerosis in which increased bone turnover results from mutations ablating two functional motifs of MEPE, leading to both accelerated osteoclast differentiation and enhanced mineralization and thus increased bone turnover14. In contrast with otosclerosis, they observed frameshift mutations ablating only one functional motif of MEPE in individuals with a severe craniofacial defect with thickening of the skull, potentially reflecting an unopposed increase in mineralization. Here, we report a genome-wide significant association in FinnGen between otosclerosis and the frameshift mutation rs753138805 that is likely to ablate both functional motifs in MEPE. The same mutation was identified in two families (Dutch and Belgian) and nine unrelated cases by Schrauwen and colleagues14. Reflecting a systemic effect on the skeleton, the rs753138805 variant is also associated with increased risk of leg fractures40,41. The identification of such a Finnish-enriched high-impact variant is likely possible due to a relatively recent population bottleneck event and follows similar reports for other traits42,43,44. An inherent shortcoming is that replicating variants that are rare in other populations is challenging in a GWAS setting. Yet, previous literature combined with the genetic association and immunostaining findings in this study elevate MEPE as a likely high-impact susceptibility gene for otosclerosis.
Several genes in the susceptibility loci converge on transforming growth factor beta receptor signalling pathways. TGFB1 has long been proposed as a susceptibility gene for otosclerosis, but the quality of the supporting evidence has been relatively weak17,18,19,20,21,32. Here, we report an association at the TGFB1 locus at genome-wide significance, with the strongest association for the intronic variant rs8105161.
TGFβ1, a member of the transforming growth factor beta superfamily, is a cytokine with an essential role in skeletal development and mature bone remodeling, regulating both osteoblast and osteoclast cell lineages45,46. Gain-of-function mutations in TGFB1 predispose to diaphyseal dysplasia, and mutations in other TGFβ superfamily members can cause multiple skeletal disorders of varying severity47. In the mouse inner ear, TGFβ1 is abundantly expressed in stria vascularis and supporting cells of the sensorineural epithelium48. As a secreted molecule, TGFβ1 could diffuse from these cells into the surrounding otic capsule to regulate its remodeling in a paracrine fashion. In support of this hypothesis, TGFβ inducer, called TGFBI is abundant in human inner ear fluid, called perilymph, while mRNA encoding TGFBI is localized in murine cochlear neurons and hair cells49. TGFβ1 is also produced locally within the murine otic capsule where Tgfb1 mRNA levels are significantly higher than in the parietal bone36.
Other genes relevant for TGFβ signalling include SMAD3, CD109, LTBP3, and AHSG, all of which are nearest to the lead variants in their respective loci, and RUNX2 which partly overlaps an association signal (Supplementary Table 1). Smad3 is a downstream transcription factor in the TGFβ signalling pathway; mutations in SMAD3 can cause an aneurysms-osteoarthritis syndrome with features of craniofacial and skeletal abnormalities50. RUNX2 is a transcription factor regulated by TGFβ1-signalling via both the canonical Smad and p38 MAPK pathways and has an essential role in osteoblast and chondrocyte differentiation45,46,51. Relevantly, we found RUNX2 expression in the developing but not adult otic capsule (Fig. 2). This finding is significant because otosclerosis is associated with embryonic cell rests within the otic capsule, which are unique to the human temporal bone. CD109 is a TGF-β co-receptor and negative regulator of TGF-β signalling52. Latent-transforming growth factor beta-binding protein 3 (LTBP3) can regulate the latency and activation of TGFβ1 through direct extracellular binding53. Alpha2-HS-glycoprotein/fetuin (AHSG) antagonizes TGFβ1-signalling by binding to TGFβ1 and TGFβ related bone morphogenic proteins (BMPs)54,55. AHSG can also affect mineralization directly by inhibiting calcium phosphate precipitation via formation of calcium-fetuin complexes56,57,58. Importantly, mutations in noggin, which is an inhibitor of several BMPs, are associated with multiple-synostoses syndrome whose otologic presentation mimics otosclerosis and is characterized by stapes fixation59.
In addition to signaling proteins, COL1A1 coding for the major subunit of type I collagen is a prominent candidate gene for otosclerosis11,31,32,33,60. Mutations in COL1A1 predispose to osteogenesis imperfecta, often also characterized by hearing loss. In osteogenesis imperfecta, similarly to carriers of truncating MEPE variants, increased bone turnover in the middle ear could occur in association with general skeletal fragility. While we do not observe a strong signal for COL1A1, similarly to most examined candidate genes, we report an intronic association signal in COL4A2, coding for a subunit of collagen IV, located in the basement membrane. Future mechanistic studies could examine whether COL4A2 has a structural or signaling role in otosclerosis. COL4A2 is highly conserved across species61. We are not aware of a previous association with skeletal disorders. However, mutations in COL4A3-5 genes are known to cause Alport’s syndrome, which manifests as progressive sensorineural hearing loss and nephritis. Histopathologically, Alport’s syndrome is characterized by abnormalities of the cochlear basement membrane supporting the organ of Corti and dysmorphogenesis of the organ of Corti62. These alterations are thought to affect cochlear micromechanics. While the distribution of type IV collagen alpha 1, alpha 3 and alpha 5 chains has been described in the human cochlea, the distribution of alpha 2 chain remains to be determined63. Although type IV collagens regulate BMPs in Drosophila, a similar role in humans is uncertain64.
Our results highlight several genes and signaling pathways for follow-up mechanistic studies. Genetic discovery has also increasingly preceded or guided therapeutic development. Future sequencing studies could aim to discover loss-of-function mutations within the susceptibility loci to approximate the effects of therapeutic inhibition. In the case of TGFB1, loss-of-function mutations are exceedingly rare and functional studies are likely needed to assess the effect of direct TGFβ1 inhibition26. Of note, an inhibitor of BMP type I receptor kinases has been studied for the treatment of ectopic ossification; however, in our meta-analysis, its targets (coded by ACVR1 and BMPR1A) were not significantly associated with otosclerosis65. Reflecting the heterogeneity of conditions associated with different TGFβ superfamily genes, therapeutic intervention may need to be precisely tailored to each condition45.
Although we present the largest GWAS study of otosclerosis to date, our study has limitations. The identification of otosclerosis cases is based on ICD diagnoses, which we could not verify with audiometric or imaging data. The prevalence of otosclerosis was slightly higher in FinnGen and EstBB than the reported prevalence in Western populations. This may be explained by the ascertainment of cases from hospital and specialist outpatient settings for these biobanks. The prevalence of stapes procedures among cases in FinnGen and EstBB is concordant with clinical experience. Case ascertainment in UKBB was more reliant on self-reported and procedure-based data compared with FinnGen and EstBB. Statistical colocalization analyses relied on expression data from non-bone tissues; associations in bone could differ. Although nearest genes to lead variants are causal more often than expected by chance, the physical proximity of lead variants with biologically relevant genes does not prove causation; identified and unidentified variants in the loci can exert their effects through yet unknown mechanisms67.
In summary, we determine a polygenic basis to otosclerosis with 27 genome-wide significant susceptibility loci. Analysis of the association loci suggests potential avenues for understanding disease pathophysiology through genes involved in bone remodeling, mineralization, and basement membrane collagen composition. In particular, our results highlight several genes involved in TGFβ signalling for follow-up studies.
Methods
Cohort descriptions
We identified individuals with ICD-based otosclerosis diagnoses from three national biobank-based cohorts: The Finnish FinnGen cohort, the Estonian Biobank (EstBB) and the UK Biobank (UKBB). Written informed consent was obtained from all study participants at recruitment.
The FinnGen data used here comprise 250,844 individuals from FinnGen Data Freeze 6 (https://www.finngen.fi/en)66. The data were linked by unique national personal identification numbers to the national hospital discharge registry (available from 1968) and the specialist outpatient registry (available from 1998). Data comprised in FinnGen Data Freeze 6 are administered by regional biobanks (Auria Biobank, Biobank of Central Finland, Biobank of Eastern Finland, Borealis Biobank, Helsinki Biobank, Tampere Biobank), the Blood Service Biobank, the Terveystalo Biobank, and biobanks administered by the Finnish Institute for Health and Welfare (THL) for the following studies: Botnia, Corogene, FinHealth 2017, FinIPF, FINRISK 1992–2012, GeneRisk, Health 2000, Health 2011, Kuusamo, Migraine, Super, T1D, and Twins). Patients and control subjects in FinnGen provided informed consent for biobank research, based on the Finnish Biobank Act. Alternatively, older research cohorts, collected prior the start of FinnGen (in August 2017), were collected based on study-specific consents and later transferred to the Finnish biobanks after approval by Fimea, the National Supervisory Authority for Welfare and Health. Recruitment protocols followed the biobank protocols approved by Fimea. The Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (HUS) approved the FinnGen study protocol Nr HUS/990/2017.
The FinnGen study is approved by Finnish Institute for Health and Welfare (permit numbers: THL/2031/6.02.00/2017, THL/1101/5.05.00/2017, THL/341/6.02.00/2018, THL/2222/6.02.00/2018, THL/283/6.02.00/2019, THL/1721/5.05.00/2019, THL/1524/5.05.00/2020, and THL/2364/14.02/2020), Digital and population data service agency (permit numbers: VRK43431/2017-3, VRK/6909/2018-3, VRK/4415/2019-3), the Social Insurance Institution (permit numbers: KELA 58/522/2017, KELA 131/522/2018, KELA 70/522/2019, KELA 98/522/2019, KELA 138/522/2019, KELA 2/522/2020, KELA 16/522/2020 and Statistics Finland (permit numbers: TK-53-1041-17 and TK-53-90-20).
The Biobank Access Decisions for FinnGen samples and data utilized in FinnGen Data Freeze 6 include: THL Biobank BB2017_55, BB2017_111, BB2018_19, BB_2018_34, BB_2018_67, BB2018_71, BB2019_7, BB2019_8, BB2019_26, BB2020_1, Finnish Red Cross Blood Service Biobank 7.12.2017, Helsinki Biobank HUS/359/2017, Auria Biobank AB17-5154, Biobank Borealis of Northern Finland_2017_1013, Biobank of Eastern Finland 1186/2018, Finnish Clinical Biobank Tampere MH0004, Central Finland Biobank 1-2017, and Terveystalo Biobank STB 2018001.
EstBB is a population-based cohort of 200,000 participants with a rich variety of phenotypic and health-related information collected for each individual68. At recruitment, participants have signed a consent to allow follow-up linkage of their electronic health records (EHR), thereby providing a longitudinal collection of phenotypic information. EstBB allows access to the records of the national Health Insurance Fund Treatment Bills (from 2004), Tartu University Hospital (from 2008), and North Estonia Medical Center (from 2005). For every participant there is information on diagnoses in ICD-10 coding and drug dispensing data, including drug ATC codes, prescription status and purchase date (if available). The study has obtained approval from the Ethics Review Committee on Human Research of the University of Tartu.
UKBB comprises phenotype data from 500,000 volunteer participants from the UK population aged between 40 and 69 years during recruitment in 2006–201069. Data for all participants have been linked with national Hospital Episode Statistics. UK Biobank has approval from the North West Multi-centre Research Ethics Committee (MREC) as a Research Tissue Bank (RTB) approval. The analyses for this study have been conducted under UK Biobank Application Numbers 31063 and 22627.
Identification of otosclerosis cases
Case status was assigned for individuals with any ICD-10 H80* code, the ICD-9 code 387, or the ICD-8 code 386 (Table 1), jointly corresponding to the Phecode 383. In FinnGen, only ICD codes registered in the specialty care setting were used for case definition. In UKBB, due to a large number of likely cases without ICD codes, cases were additionally ascertained based on self-reported otosclerosis diagnoses (code 20002), self-reported stapedectomy operation codes (code 20004), and the OPCS4 codes D171 (stapedectomy) and D172 (revision of stapedectomy). No self-reported data was available in FinnGen or EstBB. In FinnGen, stapes procedures were identified based on the Nomesco codes DDA00 (stapedotomy) and DDB00 (stapedectomy), and in EstBB, based on the national health insurance treatment service code 61006 (stapedotomy). Individuals not meeting these otosclerosis case definitions were designated as controls.
Genotyping and imputation of variants
FinnGen samples were genotyped using Illumina and Affymetrix arrays (Illumina Inc., San Diego, and Thermo Fisher Scientific, Santa Clara, CA, USA). Genotype imputation was performed using a population-specific SISu v3 imputation reference panel comprised of 3,775 whole genomes as described in a public protocol (https://www.protocols.io/view/genotype-imputation-workflow-v3-0-xbgfijw). We validated the imputation of the rs753138805 variant in MEPE within the Migraine Family subcohort of FinnGen. The primers were designed with the primer3 software v1.1.4. The left (forward) primer sequence was TCCATGAAACCTGATTTGACCA and right (reverse) primer sequence was CCCAGGAGTTTAATCGCAGT. Among 65 individuals determined as heterozygous for rs753138805 by imputation, we confirmed the genotype by Sanger sequencing in 56 individuals (86%). Five carriers predicted to be homozygous for the reference allele were examined as negative controls and their genotypes were confirmed by Sanger sequencing.
The samples from the Estonian Biobank were genotyped at the Genotyping Core Facility of the Institute of Genomics, University of Tartu using the Global Screening Array (GSAv1.0, GSAv2.0, and GSAv2.0_EST) from Illumina. Altogether 206,448 samples were genotyped and PLINK format files were exported using GenomeStudio v2.0.470. Individuals were excluded from the analysis if their call-rate was < 95% or if the sex defined based on heterozygosity of the X chromosome did not match the sex in phenotype data. Variants were excluded if the call-rate was < 95% or HWE p-value was < 1e-4 (autosomal variants only). Variant positions were updated to genome build 37 and all alleles were switched to the TOP strand using tools and reference files provided at https://www.well.ox.ac.uk/~wrayner/strand/. After QC the dataset contained 202,910 samples for imputation. Before imputation variants with MAF < 1% and indels were removed. Prephasing was done using the Eagle v2.3 software71. The number of conditioning haplotypes Eagle2 uses when phasing each sample was set to: --Kpbwt=20000. Imputation was done using Beagle v.28Sep18.793 with effective population size ne=20,00072. An Estonian population specific imputation reference of 2297 WGS samples was used73.
UKBB samples have been genotyped and imputed and released for research use previously69. In brief, genotyping of stored blood samples was carried out by Affymetrix Research Services Laboratory, and Affymetrix applied a custom genotype calling pipeline and quality filtering. Approximately 850,000 variants were directly genotyped. After imputation of over 90 million variants, a total of 97,059,328 UKBB version 3 imputed variants were released and were included in this study prior to further quality controls detailed below.
Statistical analysis
GWAS for the individual study cohorts was performed using a generalized mixed model with the saddlepoint approximation using SAIGE v0.20, using a kinship matrix as a random effect and covariates as fixed effects74. For all cohorts, variants with a minor allele frequency less than 0.1% were excluded.
In FinnGen, samples from individuals with non-Finnish ancestry and twin/duplicate samples were excluded, and GWAS was performed for 1,563 cases and 249,281 controls. Age, sex, 10 PCs and the genotyping batch (for batches with at least 10 cases and controls) were used as covariates. Results were filtered to variants with imputation INFO score > 0.6. In addition to the primary GWAS, we performed additional sensitivity analyses in FinnGen to evaluate the effect of gender and control selection (Supplementary Fig. 6 and Supplementary Data 24-26). Separate GWAS were conducted for women (1002 cases and 140,222 controls) and men (561 cases and 109,059 controls) to evaluate potential differences in effect directions, which were not observed. To evaluate the potential effect of undiagnosed cases among controls, GWAS were also performed comparing all 1,563 cases with controls filtered to include only individuals over age 65 (110,166 controls), individuals without any ICD-based diagnosis of hearing loss (231,502 controls) and individuals over age 65 without any ICD-based diagnosis of hearing loss (96,564 controls). Due to the similarity of the GWAS signals for different control definitions, we continued with the initial control definition for all subsequent analyses in each cohort.
In EstBB, a GWAS was performed on 985 cases and 196,516 controls of European ancestry including related individuals and adjusting for the first 10 PCs of the genotype matrix, as well as for birth year and sex.
In UKBB, European ancestry classification and genomic analyses were performed identically to the approach in the Pan UKBB study (https://pan.ukbb.broadinstitute.org/docs/qc). Case and control selection were refined specifically for this study. Samples of European ancestry were identified via a two-stage approach. First, continental ancestry was assigned based on principal component (PC) analysis on unrelated individuals from a combined 1000 Genomes and Human Genome Diversity Panel reference dataset. PC loadings were used to project UKBB individuals into the same PC space. A random forest classifier was trained based on continental ancestry meta-data and top 6 PCs from the reference training data. Second, assigned ancestry classifications were further refined by pruning outliers within each continental assignment by plotting histograms of individual distances from population centroids calculated across 10 PCs. From all version 3 imputed variants, a total of 29,865,259 with INFO score > 0.8 and an allele count of at least 20 in each population were retained for the GWAS with SAIGE. Age, sex, age*sex, age2, age2*sex and the first 10 PCs were used as GWAS covariates.
Approximate estimates for the narrow sense heritability of otosclerosis in each cohort and the subsequent meta-analysis were obtained and compared using three different summary statistics based methods: 1) LD Score Regression (LDSC) v1.0.1, and 2) BLK-LDAK and 3) LDAK-Thin of the SumHer software in LDAK v5.023,24,75. We used summary statistics separately from the meta-analysis and each cohort, restricting the analyses to variants present in HapMap3 (https://www.sanger.ac.uk/resources/downloads/human/hapmap3.html)76. For the BLK-LDAK and LDAK-Thin models, we used the European tagging files based on UKBB (http://dougspeed.com/pre-computed-tagging-files/), and for LDSC we used LD Scores computed using 1000 Genomes European data (https://data.broadinstitute.org/alkesgroup/LDSCORE/eur_w_ld_chr.tar.bz2). For the liability transformations, a population prevalence approximation of 0.3% was used.
Variant positions in the UKBB and EstBB summary statistics were lifted from GRCh37 to GRCh38 using the liftOver v1.12 Bioconductor R package77. To assess similarity of genetic effects between cohorts, we used the cross-trait LD Score Regression (LDSC) v1.0.1 to calculate pairwise genetic correlations (rg) based on summary statistics from each cohort for variants present in HapMap323,24.
Summary statistics from the individual cohorts for 13,615,309 variants present in at least two cohorts with a cross-cohort minor allele frequency > 0.1% and imputation INFO score ≥ 0.7 were combined using an inverse-variance weighted fixed-effect meta-analysis with GWAMA v2.2.278. The following analyses are based on summary statistics from the meta-analysis unless otherwise specified.
Characterization of association loci
For the individual cohorts and meta-analysis, we merged genome-wide significant variants within 1.5 Mb of each other into association loci. The lead variants from these loci were not in LD with each other (r2 < 1 × 10−4 for all variant pair comparisons in FinnGen). We annotated the lead variants by mapping their physical position to genes and consequences using the Ensembl Variant Effect Predictor (VEP) based on the GRCh38 genome build (https://useast.ensembl.org/info/docs/tools/vep/index.html)79. In addition, we annotated all genome-wide significant variants and all variants in high LD (r2 > 0.6) with the lead variants from the meta-analysis using VEP. As the highest number of association loci was observed in FinnGen, we estimated LD in the FinnGen cohort using PLINK v1.0780,81. LocusZoom was used to visualize the association loci and the regions surrounding other genes of interest82.
Fine-mapping
Based on otosclerosis summary statistics and linkage disequilibirium information from FinnGen, we fine-mapped all regions where the lead variant reached a p-value of < 1 × 10−6 in FinnGen using SuSiE27. In-sample dosage LD was computed using LDStore2 for each fine-mapping region. We used a 3 Mb window (±1.5 Mb) around each lead variant (merging overlapping regions) with 10 as the maximum number of causal variants in a locus.
Phenome-wide association analyses and genetic correlations
For all 27 lead variants from genome-wide significant association loci in the meta-analysis, we queried the GWAS Atlas database (https://atlas.ctglab.nl) for significant associations among 4756 reported traits83. GWAS Atlas is a comprehensive database of publicly available GWAS summary statistics. We catalogued traits which were significantly associated with the lead variants at the Bonferroni-adjusted threshold of p < 1.05 × 10−5.
We also evaluated potential directional concordance or discordance of genetic effect estimates for recurring traits and otosclerosis using binomial tests with the null hypothesis that directional concordance and discordance are equally likely. All previously reported associations reaching nominal significance (p-value < 0.05) in GWAS Atlas were included, and seven traits which were associated with at least ten otosclerosis GWAS lead variants were identified (Supplementary Data 15). A Bonferroni-corrected p-value threshold of 0.05/7 was used to evaluate statistical significance in the binomial tests.
For the MEPE frameshift variant observable only in FinnGen and not included in GWAS Atlas, we performed a phenome-wide association analysis of 2861 registry-based disease endpoints in FinnGen66. A Bonferroni-corrected threshold α = 0.05/2861) was used to establish significance. GWAS for the endpoints was performed similarly to the GWAS for otosclerosis. The study participants were linked with national registries covering the whole population for hospital discharges (data available since 1968), deaths (1969–), outpatient specialist appointments (1998–), cancers (1953–), and medication reimbursements (1995–). Disease endpoints were collated based on International Classification of Diseases (ICD) codes (revisions 8–10), International Classification of Diseases for Oncology (ICD-O) third edition codes, NOMESCO procedure codes, Finnish-specific Social Insurance Institute (KELA) drug reimbursement codes, and drug-specific ATC-codes. In addition, specific clinical endpoints combining relevant comorbidities and exclusion criteria have been curated in coordination with clinical expert groups. Currently available FinnGen phenotype definitions are available online at https://www.finngen.fi/en/researchers/clinical-endpoints.
Following frequently observed associations of the meta-analysis lead variants in the phenome-wide association analysis, we tested the genetic correlations of otosclerosis with all available bone, anthropometric and lung traits in LDHub, a centralized database of summary-level GWAS results. Genetic correlation analyses were conducted using the automated pipeline after filtering the meta-analysis summary statistics to the HapMap3 variants recommended by the authors (v1.9.3.; http://ldsc.broadinstitute.org/ldhub/)23,24,29. A Bonferroni-corrected p-value threshold (α = 0.05/54) was used to evaluate statistical significance.
Finally, we queried the GWAS catalog (version 1.0.2) and the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar; accessed on 14 April 2021) for previous associations with rare diseases for a) any of the 1452 variants associated with otosclerosis at genome-wide significance, and b) any variants in the association loci which were associated with otosclerosis at nominal significance (p < 0.05).
Gene and gene set -based analyses
We used MAGMA v1.08 to identify genes and gene sets associated with otosclerosis based on effect estimates from the meta-analysis84. Variants were mapped to 18,877 genes based on their RefSNP numbers. We performed a gene-based analysis using the default SNPwise-mean model and a Bonferroni-corrected p-value threshold (α = 0.05/18,877). Based on the results from the gene-based analysis, we then performed a competitive gene set based analysis using 10,182 GO term based gene sets downloaded from the Molecular Signature Database v7.1 (http://www.gsea-msigdb.org/gsea/msigdb), using a Bonferroni-corrected p-value threshold (α = 0.05/10,182)85. Set-specific QQ plots were produced by permutation analysis to evaluate the influence of outliers (Supplementary Fig. 7).
We performed a separate GO Biological Process (BP) enrichment analysis for the 27 protein-coding genes nearest to the lead variants from the meta-analysis. The analysis was performed using the online tool provided by the Gene Ontology Consortium connected to the PANTHER classification system (http://geneontology.org/docs/go-enrichment-analysis/ and http://pantherdb.org). Enriched biological processes with a false discovery rate (FDR) corrected p-value under 0.05 are reported.
Colocalization
We performed a colocalization analysis similar to the probabilistic method for integrating GWAS and eQTL data presented in eCAVIAR86. We used expression data from the EMBL-EBI eQTL Catalogue (https://www.ebi.ac.uk/eqtl/) for 24 tissues/cell types as fine-mapped previously by Alasoo and Keriamov (https://kauralasoo.github.io)87. We calculate the causal posterior probability (CLPP) similar to Hormozdiari et al.86. In addition, we compute another colocalization metric, the causal posterior agreement (CLPA), that is independent of the size of the credible sets. For variants i intersecting in phenotype 1 (p1) and phenotype 2 (p2), where vector x contains the posterior inclusion probability for p1 and vector y for p2, CLPAk = Σi∈CSkmin(xi,yi) if i is contained in p1 and p2 (0 otherwise). CLPP represents the probability that the same locus is causal in two studies and CLPA represents the agreement of fine-mapping results between two studies. We employed a causal posterior probability cutoff of ≥ 0.1%.
Annotation of genetic loci based on previous studies
We reviewed genes in immediate proximity with the lead variants from the GWAS meta-analysis (nearest, 2nd nearest and 3rd nearest protein-coding genes) for previous reports on biological function and disease associations (Supplementary Table 1). The biological functions of proteins were evaluated based on UniProt and followed up with literature review. ClinVar and GWAS Catalog were queried for associations with severe skeletal or dental diseases. Mouse knockout data were queried using PubMed searches and available International Mouse Phenotyping Consortium (IMPC) data (https://www.mousephenotype.org; accessed April 25 2022) using the consortium’s recommended p-value threshold of 0.0001 to correct for multiple comparisons88,89.
Immunohistochemistry
Pregnant CD1 mice were obtained from Charles River Laboratories, Wilmington, CA, and were housed at 20–22 °C ambient temperature with 30–70% humidity, a 12-hour light/dark cycle, and food and water available ad libitum. All procedures were approved by the Administrative Panel on Laboratory Animal Care (APLAC) at Stanford University according to the National Institutes of Health guidelines for animal care (protocol #33998). Postnatal day 2 (P2) and 6 (P6) mice of both sexes were sacrificed, decapitated, and their temporal bones extracted. The ossicles were removed with fine forceps, the round window membrane was punctured, and the cochlea was flushed with 4% paraformaldehyde (PFA) fixative, and decalcified in 0.12 M EDTA for 24 h. Postnatal day 12 (P12) and adult, 3 month old mice were anesthetized with Ketamine (100 mg/kg) and Xylazine (10 mg/kg) and perfused intracardially with 4% PFA. Their temporal bones were collected, and inner ears were perfused with 4% PFA as described above. After decalcified in 0.12 M EDTA for 72 h, cochleae of all ages were cryoprotected in 30% sucrose, embedded in optimal cutting temperature (O.C.T.) compound, snap frozen on dry ice, and stored at −80 °C before further processing. O.C.T. blocks were sectioned on a Leica cryostat at 12 µm sections, mounted on glass slides, and stored at −20 °C. For immunofluorescent staining, tissue sections were washed with PBS three times, blocked with 5% NHS and 0.3% TX-100 in PBS for 1 h, and immunostained overnight at room temperature with the following primary antibodies diluted in 1% NHS and 0.3% TX-100: rabbit polyclonal anti-MEPE (Kerafast, Boston, MA, #ENH086-FP, 1:400;) and rabbit monoclonal RUNX2 (Cell Signaling Technology, Danvers, MA, clone D1L7F, #12556, 1:200)90. Sections were then washed in PBS and incubated with goat anti-rabbit Alexa Fluor 488 antibodies (Thermo Fisher Scientific, Waltham, MA, #A-11008, 1:500). The nuclei were counterstained with DAPI solution (Thermo Fisher Scientific, Waltham, MA, #62248, 1: 10000). Sections were imaged on a Zeiss LSM 880 confocal microscope (Jena, Germany). For immunoperoxidase staining, sections were prepared by the Stanford animal histopathology core’s standard staining process. The sections were washed and blocked with avidin, biotin, and 3% goat serum in TBS-Tween. Slides were incubated overnight with primary antibodies, endogenous peroxidases were inactivated with 3% hydrogen peroxide solution, incubated with goat anti-rabbit IgG (Abcam, Cambridge, UK, #ab64256, 5 µg/mL), washed, and incubated with horseradish peroxidase-conjugated streptavidin (Thermo Fisher Scientific, Waltham, MA, # N100, 1:1000) for signal amplification, and stained with DAB chromogen and hematoxylin. Slides were imaged on a Zeiss (Jena, Germany) AxioImager widefield microscope. As a negative control, primary antibodies were omitted from the staining protocol, resulting in no specific signal. As a positive control, previously validated, unrelated primary antibodies gave rise to different specific patterns of expression. Representative images from N = 3 animals of each age group are presented.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The meta-analysis summary statistics generated in this study have been deposited in the NHGRI-EBI GWAS Catalog under the accession code GCST90129575. Cohort-level summary statistics from FinnGen are publicly available (https://r6.finngen.fi/pheno/H8_OTOSCLE). Individual-level genotypes and register data from FinnGen participants can be accessed by approved researchers via the Fingenious portal (https://site.fingenious.fi/en/) hosted by the Finnish Biobank Cooperative FinBB (https://finbb.fi/en/). Data from the UK Biobank are available by application to all bone fine researchers in the public interest (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). The individual-level Estonian Biobank data are available under restricted access administered by the Estonian Genome Center of the University of Tartu (EGCUT) in accordance with the regulations of the Estonian Human Genes Research Act; access can be obtained by application at www.biobank.ee.
Code availability
No new algorithms were generated as part of this study. Previously published software were used to generate the analyses as detailed in the Methods section, and exact commands with parameters can be made available upon request.
References
Quesnel, A. M., Ishai, R. & McKenna, M. J. Otosclerosis: Temporal Bone Pathology. Otolaryngologic Clin. North Am. 51, 291–303 (2018).
Babcock, T. A. & Liu, X. Z. Otosclerosis: From Genetics to Molecular Biology. Otolaryngologic Clin. North Am. 51, 305–318 (2018).
Declau, F. et al. Prevalence of otosclerosis in an unselected series of temporal bones. Otol. Neurotol. 22, 596–602 (2001).
Declau, F. et al. Prevalence of histologic otosclerosis: an unbiased temporal bone study in Caucasians. Adv. oto-rhino-Laryngol. 65, 6–16 (2007).
House, H. P., Hansen, M. R., Al Dakhail, A. A. & House, J. W. Stapedectomy versus stapedotomy: comparison of results with long-term follow-up. Laryngoscope 112, 2046–2050 (2002).
Del Bo, M., Zaghis, A. & Ambrosetti, U. Some observations concerning 200 stapedectomies: fifteen years postoperatively. Laryngoscope 97, 1211–1213 (1987).
Cawthorne, T. Otosclerosis. J. Laryngol. Otol. 69, 437–456 (1955).
Morrison, A. W. Genetic factors in otosclerosis. Ann. R. Coll. Surg. Engl. 41, 202–237 (1967).
Moumoulidis, I., Axon, P., Baguley, D. & Reid, E. A review on the genetics of otosclerosis. Clin. Otolaryngol. 32, 239–247 (2007).
Larsson, A. Otosclerosis. A genetic and clinical study. Acta Otolaryngol. Suppl. 154, 1–86 (1960).
Bittermann, A. J. et al. An introduction of genetics in otosclerosis: a systematic review. Otolaryngol.−Head. Neck Surg.: Off. J. Am. Acad. Otolaryngol.-Head. Neck Surg. 150, 34–39 (2014).
Valgaeren, H. et al. Insufficient evidence for a role of SERPINF1 in otosclerosis. Mol. Genet. Genomics 294, 1001–1006 (2019).
Ziff, J. L. et al. Mutations and altered expression of SERPINF1 in patients with familial otosclerosis. Hum. Mol. Genet. 25, 2393–2403 (2016).
Schrauwen, I. et al. Variants affecting diverse domains of MEPE are associated with two distinct bone disorders, a craniofacial bone defect and otosclerosis. Genet. Med.: Off. J. Am. Coll. Med. Genet. 21, 1199–1208 (2019).
Højland, A. T. et al. A wide range of protective and predisposing variants in aggrecan influence the susceptibility for otosclerosis. Hum. Genet. https://doi.org/10.1007/s00439-021-02334-8 (2021).
Abdelfatah, N. et al. A pathogenic deletion in Forkhead Box L1 (FOXL1) identifies the first otosclerosis (OTSC) gene. Hum. Genet., https://doi.org/10.1007/s00439-021-02381-1 (2021).
Schrauwen, I. et al. A genome-wide analysis identifies genetic variants in the RELN gene associated with otosclerosis. Am. J. Hum. Genet. 84, 328–338 (2009).
Mowat, A. J. et al. Evidence of distinct RELN and TGFB1 genetic associations in familial and non-familial otosclerosis in a British population. Hum. Genet 137, 357–363 (2018).
Priyadarshi, S., Hansdah, K., Ray, C. S., Biswal, N. C. & Ramchander, P. V. Otosclerosis associated with a de novo mutation -832G > A in the TGFB1 gene promoter causing a decreased expression level. Sci. Rep. 6, 29572 (2016).
Priyadarshi, S. et al. Genetic association and gene expression profiles of TGFB1 and the contribution of TGFB1 to otosclerosis susceptibility. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 28, 2490–2497 (2013).
Thys, M. et al. Detection of rare nonsynonymous variants in TGFB1 in otosclerosis patients. Ann. Hum. Genet. 73, 171–175 (2009).
Schrauwen, I. et al. Association of bone morphogenetic proteins with otosclerosis. J. Bone Miner. Res. 23, 507–516 (2008).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Pauw, R. J. et al. Phenotype description of a Dutch otosclerosis family with suggestive linkage to OTSC7. Am. J. Med. Genet. Part A 143A, 1613–1622 (2007).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Wang, G., Sarkar, A., Carbonetto, P. & Stephens, M. A simple new approach to variable selection in regression, with application to genetic fine mapping. J. R. Stat. Soc.: Ser. B (Stat. Methodol.) 82, 1273–1300 (2020).
Cleiren, E. et al. Albers-Schönberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum. Mol. Genet. 10, 2861–2867 (2001).
Zheng, J. et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinforma. (Oxf., Engl.) 33, 272–279 (2016).
Nakajima, M. et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 46, 1012–1016 (2014).
Schrauwen, I. et al. COL1A1 association and otosclerosis: a meta-analysis. Am. J. Med. Genet. Part A 158A, 1066–1070 (2012).
Khalfallah, A. et al. Association of COL1A1 and TGFB1 polymorphisms with otosclerosis in a tunisian population. Ann. Hum. Genet. 75, 598–604 (2011).
Chen, W. et al. Single-nucleotide polymorphisms in the COL1A1 regulatory regions are associated with otosclerosis. Clin. Genet. 71, 406–414 (2007).
Frisch, T., Sørensen, M. S., Overgaard, S. & Bretlau, P. Estimation of volume referent bone turnover in the otic capsule after sequential point labeling. Ann. Otol. Rhinol. Laryngol. 109, 33–39 (2000).
Zehnder, A. F., Kristiansen, A. G., Adams, J. C., Merchant, S. N. & McKenna, M. J. Osteoprotegerin in the inner ear may inhibit bone remodeling in the otic capsule. Laryngoscope 115, 172–177 (2005).
Stankovic, K. M. et al. Differences in gene expression between the otic capsule and other bones. Hear Res. 265, 83–89 (2010).
Rudic, M. et al. The pathophysiology of otosclerosis: review of current research. Hearing Res. 330, 51–56 (2015).
Kao, S. Y. et al. Loss of osteoprotegerin expression in the inner ear causes degeneration of the cochlear nerve and sensorineural hearing loss. Neurobiol. Dis. 56, 25–33 (2013).
Kao, S. Y. et al. Postnatal expression and possible function of RANK and RANKL in the murine inner ear. Bone 145, 115837 (2021).
Surakka, I. et al. MEPE loss-of-function variant associates with decreased bone mineral density and increased fracture risk. Nat. Commun. 11, 4093 (2020).
Van Hout, C. V. et al. Exome sequencing and characterization of 49,960 individuals in the UK Biobank. Nature 586, 749–756 (2020).
Helkkula, P. et al. ANGPTL8 protein-truncating variant associated with lower serum triglycerides and risk of coronary disease. PLoS Genet. 17, e1009501 (2021).
Lim, E. T. et al. Distribution and medical impact of loss-of-function variants in the finnish founder population. PLoS Genet. 10, e1004494 (2014).
Manning, A. et al. A low-frequency inactivating AKT2 variant enriched in the finnish population is associated with fasting insulin levels and type 2 diabetes risk. Diabetes 66, 2019–2032 (2017).
Wu, M., Chen, G. & Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 4, 16009 (2016).
Crane, J. L. & Cao, X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J. Clin. Invest. 124, 466–472 (2014).
Kinoshita, A. et al. Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nat. Genet. 26, 19–20 (2000).
Bas, E., Anwar, M. R. & Van De Water, T. R. TGF β-1 and WNT signaling pathways collaboration associated with cochlear implantation trauma-induced fibrosis. Anat. Rec. (Hoboken) 303, 608–618 (2020).
Lin, H.-C., Ren, Y., Lysaght, A. C., Kao, S.-Y. & Stankovic, K. M. Proteome of normal human perilymph and perilymph from people with disabling vertigo. PLoS ONE 14, e0218292 (2019).
van de Laar, I. M. B. H. et al. Phenotypic spectrum of the SMAD3-related aneurysms–osteoarthritis syndrome. J. Med. Genet. 49, 47–57 (2012).
Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20071694 (2019).
Bizet, A. A. et al. CD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and function. J. Cell. Biochem. 113, 238–246 (2012).
Robertson, I. B. et al. Latent TGF-β-binding proteins. Matrix Biol.: J. Int. Soc. Matrix Biol. 47, 44–53 (2015).
Demetriou, M., Binkert, C., Sukhu, B., Tenenbaum, H. C. & Dennis, J. W. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J. Biol. Chem. 271, 12755–12761 (1996).
Szweras, M. et al. alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J. Biol. Chem. 277, 19991–19997 (2002).
Schinke, T. et al. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J. Biol. Chem. 271, 20789–20796 (1996).
Heiss, A. et al. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 278, 13333–13341 (2003).
Price, P. A. & Lim, J. E. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J. Biol. Chem. 278, 22144–22152 (2003).
Brown, D. J. et al. Autosomal dominant stapes ankylosis with broad thumbs and toes, hyperopia, and skeletal anomalies is caused by heterozygous nonsense and frameshift mutations in NOG, the gene encoding noggin. Am. J. Hum. Genet. 71, 618–624 (2002).
McKenna, M. J., Kristiansen, A. G., Bartley, M. L., Rogus, J. J. & Haines, J. L. Association of COL1A1 and otosclerosis: evidence for a shared genetic etiology with mild osteogenesis imperfecta. Am. J. Otol. 19, 604–610 (1998).
Meuwissen, M. E. et al. The expanding phenotype of COL4A1 and COL4A2 mutations: clinical data on 13 newly identified families and a review of the literature. Genet. Med.: Off. J. Am. Coll. Med. Genet. 17, 843–853 (2015).
Merchant, S. N. et al. Temporal bone histopathology in alport syndrome. Laryngoscope 114, 1609–1618 (2004).
Zehnder, A. F. et al. Distribution of type IV collagen in the cochlea in Alport syndrome. Arch. Otolaryngol. Head. Neck Surg. 131, 1007–1013 (2005).
Wang, X., Harris, R. E., Bayston, L. J. & Ashe, H. L. Type IV collagens regulate BMP signalling in Drosophila. Nature 455, 72–77 (2008).
Yu, P. B. et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 14, 1363–1369 (2008).
Kurki, M. I. et al. FinnGen provides unique genetic insights from a well phenotyped isolated population. Nature https://doi.org/10.1038/s41586-022-05473-8 (2023).
Stacey, D. et al. ProGeM: a framework for the prioritization of candidate causal genes at molecular quantitative trait loci. Nucleic acids Res. 47, e3 (2019).
Leitsalu, L., Alavere, H., Tammesoo, M. L., Leego, E. & Metspalu, A. Linking a population biobank with national health registries-the estonian experience. J. Personalized Med. 5, 96–106 (2015).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 7 (2015).
Loh, P. R. et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 48, 1443–1448 (2016).
Browning, S. R. & Browning, B. L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81, 1084–1097 (2007).
Mitt, M. et al. Improved imputation accuracy of rare and low-frequency variants using population-specific high-coverage WGS-based imputation reference panel. Eur. J. Hum. Genet. 25, 869–876 (2017).
Zhou, W. et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat. Genet. 50, 1335–1341 (2018).
Speed, D. & Balding, D. J. SumHer better estimates the SNP heritability of complex traits from summary statistics. Nat. Genet. 51, 277–284 (2019).
Altshuler, D. M. et al. Integrating common and rare genetic variation in diverse human populations. Nature 467, 52–58 (2010).
Bioconductor Package Maintainer (2020). liftOver: Changing genomic coordinate systems with rtracklayer::liftOver. R package version 1.12.0, https://www.bioconductor.org/help/workflows/liftOver/.
Mägi, R. & Morris, A. P. GWAMA: software for genome-wide association meta-analysis. BMC Bioinforma. 11, 288 (2010).
McLaren, W. et al. The ensembl variant effect predictor. Genome Biol. 17, 122 (2016).
PLINK v1.07. URL: https://zzz.bwh.harvard.edu/plink/.
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Boughton, A. P. et al. LocusZoom.js: interactive and embeddable visualization of genetic association study results. Bioinformatics 37, 3017–3018 (2021).
Watanabe, K. e. a. A global view of genetic architecture in human complex traits. [under preparation].
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS computational Biol. 11, e1004219 (2015).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Hormozdiari, F. et al. Colocalization of GWAS and eQTL signals detects target genes. Am. J. Hum. Genet. 99, 1245–1260 (2016).
Kerimov, N. et al. A compendium of uniformly processed human gene expression and splicing quantitative trait loci. Nat. Genet. 53, 1290–1299 (2021).
Dickinson, M. E. et al. High-throughput discovery of novel developmental phenotypes. Nature 537, 508–514 (2016).
Bowl, M. R. et al. A large scale hearing loss screen reveals an extensive unexplored genetic landscape for auditory dysfunction. Nat. Commun. 8, 886–886 (2017).
Gluhak-Heinrich, J., Pavlin, D., Yang, W., MacDougall, M. & Harris, S. E. MEPE expression in osteocytes during orthodontic tooth movement. Arch. Oral. Biol. 52, 684–690 (2007).
Acknowledgements
K.M.S. is grateful for support from the Bertarelli Foundation Professorship and Remondi foundation. A.P. was supported by Academy of Finland Centre of Excellence in Complex Disease Genetics (grant no. 312074 and 336824). S.R. was supported by Academy of Finland Centre of Excellence in Complex Disease Genetics (grant no. 312062 and 336820). J.T.R was supported by a research fellowship from the Sigrid Jusélius Foundation. A.S.H. was supported by the Academy of Finland (grant no. 321356). T.E. was supported by Estonian Research Council grant PRG1291. A. Metspalu was supported by the European Union through the European Regional Development Fund (Project No. 2014-2020.4.01.15-0012). We thank Dr. Eric Fauman for valuable comments made to the preprint version of the manuscript. We want to acknowledge all participants and investigators in the FinnGen, Estonian Biobank and UK Biobank studies. The FinnGen project is funded by two grants from Business Finland (HUS 4685/31/2016 and UH 4386/31/2016) and the following industry partners: AbbVie Inc., AstraZeneca UK Ltd, Biogen MA Inc., Bristol Myers Squibb (and Celgene Corporation & Celgene International II Sàrl), Genentech Inc., Merck Sharp & Dohme Corp, Pfizer Inc., GlaxoSmithKline Intellectual Property Development Ltd., Sanofi US Services Inc., Maze Therapeutics Inc., Janssen Biotech Inc, Novartis AG, and Boehringer Ingelheim. The following biobanks are acknowledged for delivering biobank samples to FinnGen: Auria Biobank (www.auria.fi/bmiopankki), THL Biobank (www.thl.fi/biobank), Helsinki Biobank (www.helsinginbiopankki.fi), Biobank Borealis of Northern Finland (https://www.ppshp.fi/Tutkimus-ja-opetus/Biopankki/Pages/Biobank-Borealis-briefly-in-English.aspx), Finnish Clinical Biobank Tampere (www.tays.fi/en-US/Research_and_development/Finnish_Clinical_Biobank_Tampere), Biobank of Eastern Finland (www.ita-suomenbiopankki.fi/en), Central Finland Biobank (www.ksshp.fi/fi-FI/Potilaalle/Biopankki), Finnish Red Cross Blood Service Biobank (www.veripalvelu.fi/verenluovutus/biopankkitoiminta) and Terveystalo Biobank (www.terveystalo.com/fi/Yritystietoa/Terveystalo-Biopankki/Biopankki/). All Finnish Biobanks are members of BBMRI.fi infrastructure (www.bbmri.fi). Finnish Biobank Cooperative -FINBB (https://finbb.fi/) is the coordinator of BBMRI-ERIC operations in Finland. K.Krebs and L.M. were supported by the Estonian Research Council (Grant PRG184), and the European Union through the European Regional Development Fund (Project No. 2014-2020.4.01.15-0012 GENTRANSMED).
Author information
Authors and Affiliations
Consortia
Contributions
A.P., M.J.D, A.Mäkitie, K.M.S., L.M., M.Kurki, K.J.Karczewski, J.T.R., T.K. and R.S. conceived the study and designed the analyses. J.T.R., T.K., R.S., J.K., K. Krebs, M.Kanai, K.J.Karczewski, M.Kurki, A.S.H., E.H., and P.H. conducted the analyses. P.P., T.E., A.Metspalu, R.M., M.P., S.R., L.M., H.H., J.K., A.Mäkitie and M.Kurki provided support in analysis implementation and interpretation of the results. J.T.R, T.K., R.S., A.P., A.Mäkitie, K.M.S. and R.S. wrote the paper with the participation of all the authors. All authors reviewed and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Dominic Furniss and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rämö, J.T., Kiiskinen, T., Seist, R. et al. Genome-wide screen of otosclerosis in population biobanks: 27 loci and shared associations with skeletal structure. Nat Commun 14, 157 (2023). https://doi.org/10.1038/s41467-022-32936-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32936-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.