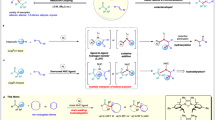
Abstract
Intermolecular addition of enols and enolates to unactivated alkynes was proved to be a simple and powerful method for carbon-carbon bond formation. Up to date, a catalytic asymmetric version of alkyne with 1,3-dicarbonyl compound has not been realized. Herein, we achieve the catalytic asymmetric intermolecular addition of 1,3-dicarbonyl compounds to unactivated 1-alkynes attributing to the synergistic activation of chiral N,N′-dioxide-indium(III) or nickel(II) Lewis acid and achiral gold(I) π-acid. A range of β-ketoamides, β-ketoesters and 1,3-diketones transform to the corresponding products with a tetra-substituted chiral center in good yields with good e.r. values. Besides, a possible catalytic cycle and a transition state model are proposed to illustrate the reaction process and the origin of chiral induction based on the experimental investigations.
Similar content being viewed by others
Introduction
The addition of carbonyl compounds without prior enolate formation to unactivated alkynes is an attractive and atom economical method for carbon–carbon bond formation1. It results in the introduction of a vinyl substituent to vicinal position of carbonyl groups, possessing an important role in organic synthesis of natural products and drugs2,3,4,5. The intramolecular type, which is known as the Conia-ene reaction, generating cycloalkene derivatives, has achieved significant progress. Besides the well-developed non-enantioselective systems6,7,8,9,10,11, catalytic asymmetric Conia-ene reactions have already been realized by synergistic hard/soft Lewis acid catalysts (e.g., Pd/Yb, Yb/Zn, Ag/La, Ag/Fe)12,13,14,15, Lewis basic amine/Lewis acid catalysts (e.g., Cu, Ag-based)16,17,18,19, and Brønsted basic amine/Lewis acid catalyst (B(C6F5)3/Zn/PMP)20. In contrast, the intermolecular reaction of 1,3-dicarbonyl compounds to unactivated 1-alkynes (Nakamura reaction) was less developed. Such a process is unviable because of the unfavorable thermodynamics that there is a high-lying LUMO of an unactivated alkyne compared to the HOMO of 1,3-dicarbonyl compounds21,22. In 2003, Nakamura et al. documented an indium-catalyzed addition of 1,3-dicarbonyl compounds to unactivated 1-alkynes23, providing an efficient synthetic route to form 2-alkenyl-1,3-dicarbonyl compounds from abundant carbon alkynes sources. After that, In(III)24,25,26,27,28, Re(I)29,30,31, Ir(I)32, Pd(0)33, Co(II)34, Mn(I)35,36, and Ru(I)-(III)37,38,39 catalytic systems were discovered, all of which were racemic reports except for only one example using substrates with chiral auxiliary40. All the above reports, the dicarbonyl compounds and alkynes need to be activated simultaneously. Beyond that, the Shi group reported a synergistic Au(I)/Ga(III) catalysis in Nakamura reaction41, in which Au(I) activated the alkynes whereas Ga(III) enhanced the acidity of the 1,3-dicarbonyl compounds42,43, affording racemic 2-alkenyl-1,3-dicarbonyl products. Generally, all the Nakamura reactions were still limited to racemic examples (Fig. 1).
Therefore, developing an efficient catalytic system to realize the asymmetric version of the Nakamura reaction is challenging but highly desirable.
Bimetallic catalysis is also promising in asymmetric catalysis44,45,46. However, one of the perceived challenges is that two distinct metals might competitively coordinate with the ligand, as well as potentially affect each other’s catalytic cycles. Recently, chiral N,Nʹ-dioxides/hard Lewis acid complexes developed by our group were found to be good partners with soft metals47,48,49,50,51 in relay catalysis systems. We envisioned that N,Nʹ-dioxide/Lewis acid complex could also be applied to synergistic catalyst system.
In this work, we developed a gold(I)/chiral N,N’-dioxide-indium(III) or nickel(II) synergistic catalyst system to realize the catalyic asymmetric Nakamura reaction of unactivated 1-alkynes with β-ketoamides, β-ketoesters, and 1,3-diketones in good reactivity and enantioselectivity. Mechanism study elucidates the process of the reaction and origin of chiral induction.
Results
Optimization of the reaction conditions
Indanone-derived β-ketoamide 1a and phenylacetylene 2a were selected as the model substrates to conduct our research. First, several cooperative catalytic systems, which showed good ability in catalytic enantioselective Conia-ene reaction, including Pd(II)/Yb(III) dual catalyst system, Zn(II)/Yb(III) catalyst system, and amine–silver system, were investigated13,16,19. But all of them gave only trace amount of product without enantioselectivities even rising the reaction temperature to 70 °C (Table 1, entries 1–3). Then chiral N,N’-dioxide ligand-metal complexes were chosen as the activators of ketoamides, in connection with AuCl∙PPh3/AgOTf for the activation of 1-alkyne. First, Sc(OTf)3 was used to coordinate with chiral N,N’-dioxide L-PiEt2 to promote the reaction under air atmosphere, the byproduct 3bb was obtained as the main product along with the desired product 3aa in 11% yield with 60:40 e.r. (entry 4). Further research showed that the reaction could possess efficiency in an absolute anaerobic condition, delivering the product 3aa in 92% yield with 60:40 e.r. (entry 5). Then Ga(OTf)3 that showed efficient catalytic activity in Shi’s report41 was used to coordinate with chiral N,N’-dioxide L-PiEt2 to promote the reaction; however, only trace of product 3aa was obtained (entry 6). To our delight, In(OTf)3 could improve the reaction activity greatly and deliver the desired product with 62:38 e.r. (entry 7). The ligand L-TQ-(S)-EPh derived from S-tetrahydroisoquinoline acid decreased the e.r. greatly (entry 8). To improve the enantioselectivity, other conditions were carefully studied. Changing the N,N’-dioxide ligand to L-PiEt2Me, which has ethyl groups at ortho-positions and methyl group at para-position of aniline, the yield could be improved to 99% (entry 9). Moreover, the addition of trace amount of H2O (entry 10) and increasing the amount of ligand L-PiEt2Me (entry 11) improved the enantioselectivity. The water might be beneficial for formation of the effective catalyst species, as well as beneficial for accelerating the enolization of 1,3-dicarbonyl compounds52. Meanwhile, the increasement of ligand might be helpful for the complete coordination with In(OTf)3, avoiding the strong background reaction caused by free metal salt. Further exploration showed that the solvent had a great influence on the reaction, when para-xylene was used as the solvent, the desired product was isolated in 98% yield with 90:10 e.r. (entry 12). The enantioselectivity enhanced into 94.5:5.5 e.r. after the concentration of 1a reduced to 0.067 mol/L by enhancing the amount of solvent (entry 13). The steric hindrance of the ligands on [Au] catalyst was another key factor. Changing the AuCl·PPh3 into more sterically hindered XPhosAu(TA)OTf, only trace product could be obtained (entry 14). The reason might be that the bulky X-Phos cause larger steric hindrance between the [Au]-activated 1-alkyne and the chiral Lewis acid-activated 1,3-dicarboyl compound, making the reaction happen more difficultly. In comparison, other indium catalysts of the typical chiral ligands such as Pybox L3, Box L2, or CPA organocatalyst were used, the product 3aa was obtained in low yield with poor e.r. value (entries 15–17).
Substrate scope of the reaction about β-ketoamides
With the optimized reaction conditions in hand (Table 1, entry 13), the substrate scope was then evaluated (Fig. 2). A variety of ketoamides 1 derived from 1-indanones with different substituents were tested. Substrates with electron-donating groups exhibited excellent yields and enantioselectivities (3ba–3ea) at 50 °C. Substrate 1f bearing an electron-withdrawing group transformed to the desired product 3fa in 98% yield with 85:15 e.r. at higher temperature (60 °C). With respect to 1-alkynes 2, when the substituents at the aromatic ring of the phenylacetylenes varied, both steric hindrance and electronic properties had little effect on the reaction (3ab–3ai). However, substrate 1,4-diethynylbenzene 2j just delivered the product 3aj in moderate yield with excellent enantioselectivity. It might be caused by the competitive coordination of the alkyne-bearing product with AuOTf∙PPh3. The thienyl-substituted alkynes (2k and 2l) were also suitable. Various aliphatic 1-alkynes (2m–2q) could also transform to the desired products in good enantioselectivities (3am–3aq); however, the yield was generally moderate. One reason is that an unidentified product generated that might be caused by In(III)-induced olefin isomerization41. Importantly, the methodology was applicable to the alkyl-alkyne derived from saccharide 2r. Next, ring structure of ketoamides was studied. The substrate 1h derived from 1-tetralone got good results (3ha–3hm), while 1i derived from 1-benzosuberone gave much lower yield and e.r.. It might be caused by steric hindrance between methylene of substrate 1i with AuOTf∙PPh3-activated 2a. Meanwhile, aliphatic substrate 1j was also tolerated, affording the product 3ja in moderate yield with good enantioselectivity. The absolute configuration of 3ae was determined to be R by X-ray crystallographic analysis and the absolute configurations of 3aa–3ac and 3ag–3ah were determined to be R by comparison of the CD spectra with that of 3ae (Fig. 2).
Unless otherwise noted, all reactions were carried out, AuCl∙PPh3/AgOTf (1:1, 5 mol%), In(OTf)3/L-PiEt2Me (1:1.2, 10 mol%), 1 (0.10 mmol) and 2 (2.0 equiv), H2O (2 μL) as additive in p-xylene (1.5 mL) at 50–70 °C for 24–120 h. Isolated yields. The e.r. values were determined by HPLC analysis on chiral column. aL-PiEt2 was used as ligand. b3.0 equiv of 2 was used. cL-PiMe3 was used as ligand.
Effect of N-protecting group
Other easily removable N-protecting groups such as N-benzyl or N-PMP were investigated by reacting with phenylacetylene (Table 2). The desired products 3ka and 3la were obtained in excellent yields but with only 56.5:43.5 e.r. and 72.5:27.5 e.r. under standard conditions. After changing the ligand to L-TQ-(S)-EPh derived from (S)-tetrahydroisoquinoline-3-carbonyl acid and (S)-phenylethanamine, adjusting the reaction temperature and solvent, the enantioselectivities were improved sharply to 90:10 e.r. and 89:11 e.r., respectively. The possible reason might be that the N-protecting group, amide moiety and backbones of the catalyst are included in discrimination of Re- and Si-face of the 1,3-dicarbonyl compounds. With L-PiEt2Me as ligand, the bulky N-tertbutylamide moiety could help to shield the Si-face of substrate 1a efficiently. On the contrary, the N-benzyl or PMP with smaller steric hindrance showed poor ability to shield the Si-face of the 1,3-dicarbonyl compounds, causing the e.r. values of the products decreased sharply in the L-PiEt2Me/In(III) system. Changing to the L-TQ-(S)-EPh/In(III) system, the steric hindrance of amide moiety and backbones of the catalyst increased, and the Si-face of the 1,3-dicarbonyl compounds could also shield better; therefore, the e.r. values of products increased.
Substrate scope of the reaction about β-ketoesters
When β-ketoesters 4a was applied in the In(OTf)3/L-PiEt2Me catalytic system, the desired product was obtained in only 10% yield with 58.5:41.5 e.r.. After extensive investigation, including use of Ni(OTf)2/L-PiMe2 as catalyst and prolonging the reaction time, the corresponding product 5aa could be obtained in 47% yield with 97.5:2.5 e.r.. The decomposition of substrate 4a is responsible for the moderate yield. More stable 4b with a smaller steric hindrance of ester protecting group could transform to the desired 5ba in 72% yield with 93.5:6.5 e.r.. Different ketoesters 4 derived from 1-indanones bearing electron-donating group or withdrawing group tolerated well. Moreover, both aliphtic alkynes and aromatic alkynes were suitable in the reaction. The yields were generally good except product 5bc. The possible reason might be that the electron-withdrawing effect of the fluorine weakened the interaction between gold catalyst and alkyne (Fig. 3).
Unless otherwise noted, all reactions were carried out, AuCl∙PPh3/AgOTf (1:1, 5 mol%), Ni(OTf)2/ligand (1:1, 10 mol%), 4 or 6 (0.10 mmol) and 2 (2.0 equiv) in p-xylene (1.0 mL) at 60 °C for 48–72 h. Isolated yields. The e.r. values were determined by HPLC analysis on chiral column. aIn(OTf)3/L-PiEt2Me = 1:1.2, 10 mol%, p-xylene (1.5 mL), 24 h. bL-PiMe2 was used as ligand.
Substrate scope of the reaction about 1,3-diketones
1,3-Diketones were next investigated. With Ni(OTf)2/L-PiEt2Me as catalyst, a variety of 1,3-diketons with no matter electron-donating group or electron-withdrawing group on the C5-position or C6-position transformed to the corresponding products 7aa–7ga in 74–96% yields with 91:9–96.5:3.5 e.r.. Besides, aromatic, aliphatic, and hetero-aromatic 1-alkynes 2 were suitable substrates (Fig. 3).
Substrate scope limitation
For acyclic β-ketoamide 8a, which without other substituent on α-position, transformed to thermodynamically stable achiral α,β-conjugated carbonyl product 9aa through olefin isomerization (Fig. 4). When acyclic β-ketoamides 8b–8i bearing methyl, phenyl, benzyl, or chlorine group on the α-position were used as the nucleophiles, the corresponding products could not be observed. The possible reason might be that the α-substitution on the 1,3-dicarbonyl compounds increased the steric hindrance when the two activated substrates participate in the reaction.
Substrate scope of α-fluoro substituted acyclic β-ketoamides
Therefore, α-fluoro substituted 8j with smaller steric hindrance and stronger acidity of α-proton was evaluated (Fig. 5). Moderate yields with good e.r. could be obtained after adjusting the ligand to L-PiEt2, increasing the reaction temperature and prolonging the reaction time. Electron-donating or electron-withdrawing substitutes on the para-position of phenyl ring were tolerated well. Generally, the 1-alkynes 2 with an electron-donating substituent led to better yields than the ones with electron-withdrawing substituents. Compared with the phenylacetylene, the more electron-rich aromatic alkynes like 2l and 2s showed better reactivities (9jl and 9js). When aliphatic 1-alkynes 2m and 2n were applied to the reaction, the products were delivered in moderate yields with good e.r. values.
Mechanism investigation
Next, the reaction mechanism was investigated (Fig. 6). Some control experiments were carried out (Fig. 6a). In the absence of AuCl∙PPh3/AgOTf or In(OTf)3/L-PiEt2Me, only trace amount of the product 3aa was detected, which indicates that the two catalysts work cooperatively. N,Nʹ-dioxide/In(OTf)3 crystal structure obtained in our previous study49 showed that a OH-bridged dinuclear indium complex forms in the presence of H2O, in which N,Nʹ-dioxide coordinates to In(III) in a tetradentate manner. Nevertheless, the investigation of relationship between the e.e. value of L-PiEt2Me and that of 3aa showed a clear linear effect (Fig. 6b), implying that the active catalytic species is likely to be the mixture of In(OTf)3 and L-PiEt2Me in a 1:1 ratio. The OH anion generated from the water in situ preparation of the chiral indium catalyst might act as a base to accelerate the enolization of 1,3-dicarbonyl compounds. In addition, the M+ peak (found: 561.1058), which corresponded to a 1:1 complex C of [Au·PPh3]+ and phenylacetylene 2a, was detected by ESI-TOF analysis in the positive-ion mode. The mixture of L-PiEt2Me, In(OTf)3, and 1a (1:1:1) in p-xylene displaying an ion at m/z 1114.4025 ([L-PiEt2Me+In3++OTf−+1a-H+] m/z calcd 1114.4036) suggested that enolized 1a coordinates to the catalyst in a 1:1 molecular ratio (Fig. 6c), which is consistent with our non-linear effect.
Proposed catalytic cycle and transition-state model
Based on the above analysis and previous work, a catalytic cycle with a possible transition state is proposed. As illustrated in Fig. 7, in L-PiEt2Me/In(OTf)3 cycle, initially, the tetradentate L-PiEt2Me coordinates to InIII to form a six-coordinate octahedral geometry complex A’ and dimer A. When ketoamide 1a was added, the basic anion of the catalytic species accelerates the deprotonation process, and the enol ion of 1a coordinates tightly to chiral indium(III) center through two oxygens to form the carbanion nucleophile intermediate B. On the other hand, as for [Au] cycle, the [Au]OTf, which is the more reactive species, would bind to the π-bond of 1-alkyne 2 in an unsymmetrical fashion to form species C. The intermediate C then reacts with the complex B to form the Au/In stabilized reactive intermediate TS, which is the origin of the stereoselectivity. Due to the Si-face of β-ketoamides, 1a is effectively shielded by the amide moiety of the catalyst, with the assistance of N-protecting group on β-ketoamide and piperidine ring on the ligand, cat1-activated π-bond of 2a approaches preferably from the Re-face to undergo an energetically favorable C–C bond forming reaction, forming the complex D with R absolute configuration at the newly formed stereogenic center. Subsequent protonation of D gives the desired product 3 and releases the two catalysts.
The in situ formed chiral N,N′-dioxide-indium(III) Lewis acid actives the 1,3-dicarbonyl compounds (intermediate B) and achiral gold(I) π-acid actives the alkyne (intermediate C) synergistically; the two intermediates reacts following subsequent protonation, giving the desired product 3 and releases the two catalysts.
Discussion
An efficient catalytic asymmetric Nakamura reaction of β-ketoamides, β-ketoesters, and 1,3-diketones with unactivated 1-alkynes is realized by developing a bimetallic synergistic catalysis. The combination of π-acid gold(I)/chiral N,Nʹ-doxide-indium(III) or nickel(II) complex enables the activation of alkyne and the efficiency and stereoselectivity of nucleophile. The steric hindrance of α-substituent on 1,3-dicarbonyl compounds and hard Lewis acid are found crucial factors for the reactivity of the reaction. In addition, the substituent of 1,3-dicarbonyl compounds, the amide moiety, and backbones of the catalyst are found to affect the enantioselectivity of the reaction greatly. A possible catalytic cycle with a transition-state model was proposed to elucidate the process of the reaction and origin of chiral induction. Further studies on hetero bimetallic synergistic or relay catalysis are underway in our laboratory.
Methods
Typical procedure for cyclic β-ketoamides involved in catalytic asymmetric reaction
A mixture of AuCl∙PPh3 (5 mol%, 2.5 mg), AgOTf (5 mol%, 1.3 mg), In(OTf)3 (10 mol%, 5.6 mg), L-PiEt2Me (12 mol%, 7.4 mg), and the N-(tert-butyl)−1-oxo-2,3-dihydro-1H-indene-2-carboxamide 1a (0.10 mmol) was added to a test tube under N2 atmosphere. Then, anhydrous para-xylene (1.5 mL) was added and the mixture was stirred at 30 °C for 30 min. Subsequently, H2O (1.1 equiv, 2.0 μL) was added under stirring at 30 °C. Five minutes later, phenylacetylene 2a (2.0 equiv, 22 μL) was added at 50 °C, and the reaction mixture continued stirring at 50 °C for 24 h. The residue was purified by flash chromatography on silica gel (petroleum ether/ethyl acetate = 15:1, v/v) to afford the desired product 3aa (98% yield, 94.5:5.5 e.r.).
Typical procedure for β-ketoesters involved in catalytic asymmetric reaction
A mixture of AuCl∙PPh3 (5 mol%, 2.5 mg), AgOTf (5 mol%, 1.3 mg), Ni(OTf)2 (10 mol%, 3.6 mg), L-PiMe3 (10 mol%, 5.6 mg), and the isopropyl 1-oxo-2,3-dihydro-1H-indene-2-carboxylate 4b (0.10 mmol) was added to a test tube under N2 atmosphere. Then, anhydrous para-xylene (1.0 mL) was added and the mixture was stirred at 30 °C for 30 min. Subsequently, phenylacetylene 2a (2.0 equiv, 22 μL) was added at 60 °C, and the reaction mixture continued stirring at 60 °C for 72 h. The residue was purified by flash chromatography on silica gel (petroleum ether/ethyl acetate = 20:1, v/v) to afford the desired product 5ba (72% yield, 93.5:6.5 e.r.).
Typical procedure for 1,3-diketones involved in catalytic asymmetric reaction
A mixture of AuCl∙PPh3 (5 mol%, 2.5 mg), AgOTf (5 mol%, 1.3 mg), Ni(OTf)2 (10 mol%, 3.6 mg), L-PiEt2Me (10 mol%, 6.2 mg), and the 2-isobutyryl-2,3-dihydro-1H-inden-1-one 6a (0.10 mmol) was added to a test tube under N2 atmosphere. Then, anhydrous para-xylene (1.0 mL) was added and the mixture was stirred at 30 °C for 30 min. Subsequently, phenylacetylene 2a (2.0 equiv, 22 μL) was added at 60 °C, and the reaction mixture continued stirring at 60 °C for 48 h. The residue was purified by flash chromatography on silica gel (petroleum ether/ethyl acetate = 25:1, v/v) to afford the desired product 7aa (96% yield, 95:5 e.r.).
Typical procedure for acyclic β-ketoamides involved in catalytic asymmetric reaction
A mixture of AuCl∙PPh3 (5 mol%, 2.5 mg), AgOTf (5 mol%, 1.3 mg), In(OTf)3 (10 mol%, 5.6 mg), L-PiEt2 (12 mol%, 7.1 mg), and the N-(tert-butyl)−2-fluoro-3-oxo-3-phenylpropanamide 8j (0.10 mmol) was added to a test tube under N2 atmosphere. Then, anhydrous para-xylene (1.5 mL) was added and the mixture was stirred at 30 °C for 30 min. Subsequently, H2O (1.1 equiv, 2.0 μL) was added under stirring at 30 °C. Five minutes later, phenylacetylene 2a (3.0 equiv, 33 μL) was added at 70 °C, and the reaction mixture continued stirring at 70 °C for 120 h. The residue was purified by flash chromatography on silica gel (petroleum ether/ethyl acetate = 6:1, v/v) to afford the desired product 9ja (46% yield, 91.5:8.5 e.r.).
Data availability
The X-ray crystallographic coordinate for structure 3ae reported in this study has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 1964558. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. All other data are available from the corresponding author upon reasonable request.
References
Dénès, F., Pérez-Luna, A. & Chemla, F. Addition of metal enolate derivatives to unactivated carbon-carbon multiple bonds. Chem. Rev. 110, 2366–2447 (2010).
Tsuji, H. et al. Indium-catalyzed cycloisomerization of ω-alkynyl-β-ketoesters into six- to fifteen-membered rings. Angew. Chem. Int. Ed. 46, 8060–8062 (2007).
Itoh, Y. et al. Efficient formation of ring structures utilizing multisite activation by indium catalysis. J. Am. Chem. Soc. 130, 17161–17167 (2008).
Zhu, S. F., Zhang, Q. H., Chen, K. & Jiang, H. F. Synergistic catalysis: metal/proton-catalyzed cyclization of alkynones toward bicyclo[3.n.1]alkanones. Angew. Chem. Int. Ed. 54, 9414–9418 (2015).
Xu, Y. et al. Organocatalytic enantioselective Conia-ene-type carbocyclization of ynamide cyclohexanones: regiodivergent synthesis of morphans and normorphans. Angew. Chem. Int. Ed. 58, 16252–16259 (2019).
Kennedy-Smith, J. J., Staben, S. T. & Toste, F. D. Gold(I)-catalyzed Conia-ene reaction of β-ketoesters with alkynes. J. Am. Chem. Soc. 126, 4526–4527 (2004).
Staben, S. T., Kennedy-Smith, J. J. & Toste, F. D. Gold(I)-catalyzed 5-endo-dig carbocyclization of acetylenic dicarbonyl compounds. Angew. Chem. Int. Ed. 43, 5350–5352 (2004).
Clarke, M. L. & France, M. B. The carbonyl ene reaction. Tetrahedron 64, 9003–9031 (2008).
Gao, Q., Zheng, B.-F., Li, J.-H. & Yang, D. Ni(II)-catalyzed Conia-ene reaction of 1,3-dicarbonyl compounds with alkynes. Org. Lett. 7, 2185–2188 (2005).
Deng, C.-L., Song, R.-J., Guo, S.-M., Wang, Z.-Q. & Li, J.-H. Copper/silver-cocatalyzed Conia-ene reaction of linear β-alkynic β-ketoesters. Org. Lett. 9, 5111–5114 (2007).
Hack, D., Blümel, M., Chauhan, P., Philipps, A. R. & Enders, D. Catalytic Conia-ene and related reactions. Chem. Soc. Rev. 44, 6059–6093 (2015).
Corkey, B. K. & Toste, F. D. Catalytic enantioselective Conia-ene reaction. J. Am. Chem. Soc. 127, 17168–17169 (2005).
Matsuzawa, A., Mashiko, T., Kumagai, N. & Shibasaki, M. La/Ag heterobimetallic cooperative catalysis: a catalytic asymmetric Conia-ene reaction. Angew. Chem. Int. Ed. 50, 7616–7619 (2011).
Suzuki, S. et al. Enantioselective 5-endo-dig carbocyclization of β-ketoesters with internal alkynes employing a four-component catalyst system. Angew. Chem. Int. Ed. 51, 4131–4135 (2012).
Horibe, T., Sakakibara, M., Hiramatsu, R., Takeda, K. & Ishihara, K. One-pot tandem Michael addition/enantioselective Conia-ene cyclization mediated by chiral iron(III)/silver(I) cooperative catalysis. Angew. Chem. Int. Ed. 59, 16470–16474 (2020).
Yang, T., Ferrali, A., Sladojevich, F., Campbell, L. & Dixon, D. J. Brønsted base/Lewis acid cooperative catalysis in the enantioselective Conia-ene reaction. J. Am. Chem. Soc. 131, 9140–9141 (2009).
Shaw, S. & White, J. D. A new iron(III)–salen catalyst for enantioselective Conia-ene carbocyclization. J. Am. Chem. Soc. 136, 13578–13581 (2014).
Blümel, M., Hack, D., Ronkartz, L., Vermeeren, C. & Enders, D. Development of an enantioselective amine-silver co-catalyzed Conia-ene reaction. Chem. Commun. 53, 3956–3959 (2017).
Fang, G. S. et al. Asymmetric cyclizations via a sequential Michael addition/Conia-ene reaction by combining multifunctional quaternary phosphonium salt and silver catalysis. Tetrahedron 75, 2706–2716 (2019).
Cao, M., Yesilcimen, A. & Wasa, M. Enantioselective Conia-ene-type cyclizations of alkynyl ketones through cooperative action of B(C6F5)3, N-alkylamine and a Zn-based catalyst. J. Am. Chem. Soc. 141, 4199–4203 (2019).
Asao, N. & Yamamoto, Y. Lewis acid-catalyzed hydrometalation and carbometalation of unactivated alkynes. Bull. Chem. Soc. Jpn. 73, 1071–1087 (2000).
Fallis, A. G. & Forgione, P. Metal mediated carbometallation of alkynes and alkenes containing adjacent heteroatoms. Tetrahedron 57, 5899–5913 (2001).
Nakamura, M., Endo, K. & Nakamura, E. Indium-catalyzed addition of active methylene compounds to 1-alkynes. J. Am. Chem. Soc. 125, 13002–13003 (2003).
Nakamura, M., Endo, K. & Nakamura, E. Indium triflate-catalyzed vinylation of β-ketoesters with acetylene gas. Org. Lett. 7, 3279–3281 (2005).
Endo, K., Hatakeyama, T., Nakamura, M. & Nakamura, E. Indium-catalyzed 2-alkenylation of 1,3-dicarbonyl compounds with unactivated alkynes. J. Am. Chem. Soc. 129, 5264–5271 (2007).
Kaneda, K., Motokura, K., Nakagiri, N., Mizugaki, T. & Jitsukawa, K. Recyclable indium catalysts for additions of 1,3-dicarbonyl compounds to unactivated alkynes affected by structure and acid strength of solid supports. Green Chem. 10, 1231–1234 (2008).
Rajesh, N. & Prajapati, D. Indium-catalyzed, novel route to β,β-disubstituted indanones via tandem Nakamura addition–hydroarylation–decarboxylation sequence. Chem. Commun. 51, 3347–3350 (2015).
Sestelo, J. P., Sarandeses, L. A., Martínez, M. M. & Alonso-Marañón, L. Indium(III) as π-acid catalyst for the electrophilic activation of carbon–carbon unsaturated systems. Org. Biomol. Chem. 16, 5733–5747 (2018).
Kuninobu, Y., Kawata, A. & Takai, K. Efficient catalytic insertion of acetylenes into a carbon−carbon single bond of nonstrained cyclic compounds under mild conditions. J. Am. Chem. Soc. 128, 11368–11369 (2006).
Horino, Y. Rhenium-catalyzed C–H and C–C bond activation. Angew. Chem. Int. Ed. 46, 2144–2146 (2007).
Murai, M., Uemura, E. & Takai, K. Amine-promoted anti-markovnikov addition of 1,3-dicarbonyl compounds with terminal alkynes under rhenium catalysis. ACS Catal. 8, 5454–5459 (2018).
Onodera, G., Kato, M., Kawano, R., Kometani, Y. & Takeuchi, R. Highly regio- and stereoselective addition of 1,3-diketones to internal alkynes catalyzed by cationic iridium complex. Org. Lett. 11, 5038–5041 (2009).
Patil, N. T. & Yamamoto, Y. Formation of a quaternary carbon center through the Pd(0)/PhCOOH-catalyzed allylation of cyclic β-keto esters and 1,3-diketones with alkynes. J. Org. Chem. 69, 6478–6481 (2004).
Verma, G. K., Rawat, M. & Rawat, D. S. Cobalt-catalysed C–C bond formation and [2+2+2] annulation of 1,3-dicarbonyls to terminal alkynes. Eur. J. Org. Chem. 2019, 4101–4104 (2019).
Kuninobu, Y. et al. Rhenium- and manganese-catalyzed insertion of alkynes into a carbon–carbon single bond of cyclic and acyclic 1,3-dicarbonyl compounds. Chem. Asian J. 4, 1424–1433 (2009).
Thorve, P. R., Guru, M. M. & Maji, B. Manganese-catalyzed divergent markovnikov addition and [2+2+2] cycloaddition of 2-carbonyl indanone with terminal alkyne. J. Org. Chem. 84, 8185–8193 (2019).
Murahashi, S.-I., Naota, T. & Nakano, Y. Ruthenium-catalyzed regioselective reactions of nitriles and 1,3-dicarbonyl compounds with terminal alkynes. Synlett. 20, 3355–3359 (2009).
Cheung, H. W., So, C. M., Pun, K. H., Zhou, Z. Y. & Lau, C. P. Hydro(trispyrazolyl)borato-ruthenium(II) diphosphinoamino complex-catalyzed addition of β-diketones to 1-alkynes and anti-markovnikov addition of secondary amines to aromatic 1-alkynes. Adv. Synth. Catal. 353, 411–425 (2011).
Pennington-Boggio, M. K., Conley, B. L. & Williams, T. J. A ruthenium-catalyzed coupling of alkynes with 1,3-diketones. J. Organomet. Chem. 716, 6–10 (2012).
Fujimoto, T., Endo, K., Tsuji, H., Nakamura, M. & Nakamura, E. Construction of a chiral quaternary carbon center by indium-catalyzed asymmetric α-alkenylation of β-ketoesters. J. Am. Chem. Soc. 130, 4492–4496 (2008).
Xi, Y. M. et al. Synergistic Au/Ga catalysis in ambient Nakamura reaction. Org. Lett. 16, 306–309 (2014).
Zheng, W.-F., Xu, Q.-J. & Kang, Q. Rhodium/Lewis acid catalyzed regioselective addition of 1,3-dicarbonyl compounds to internal alkynes. Organometallics. 36, 2323–2330 (2017).
Bhattacharjee, R., Nijamudheen, A. & Datta, A. Mechanistic insights into the synergistic catalysis by Au(I), Ga(III), and counterions in the Nakamura reaction. Org. Biomol. Chem. 13, 7412–7420 (2015).
Inamdar, S. M., Shinde, V. S. & Patil, N. T. Enantioselective cooperative catalysis. Org. Biomol. Chem. 13, 8116–8162 (2015).
Shibasaki, M., Kanai, M., Matsunaga, S. & Kumagai, N. Recent progress in asymmetric bifunctional catalysis using multimetallic systems. Acc. Chem. Res. 42, 1117–1127 (2009).
Matsunaga, S. & Shibasaki, M. Recent advances in cooperative bimetallic asymmetric catalysis: dinuclear Schiff base complexes. Chem. Commun. 50, 1044–1057 (2014).
Li, J. et al. Bimetallic gold(I)/chiral N,Nʹ-dioxide nickel(II) asymmetric relay catalysis: chemo- and enantioselective synthesis of spiroketals and spiroaminals. Angew. Chem. Int. Ed. 55, 6075–6078 (2016).
Li, J. et al. Gold(I)/chiral N,Nʹ-dioxide-nickel(II) relay catalysis for asymmetric tandem intermolecular hydroalkoxylation/Claisen rearrangement. Angew. Chem. Int. Ed. 56, 885–888 (2017).
Chen, Y. S., Dong, S. X., Xu, X., Liu, X. H. & Feng, X. M. Bimetallic rhodium(II)/indium(III) relay catalysis for tandem insertion/asymmetric Claisen rearrangement. Angew. Chem. Int. Ed. 57, 16554–16558 (2018).
Zheng, H. F. et al. Diversified Cycloisomerization/Diels–Alder reactions of 1,6-enynes through bimetallic relay asymmetric catalysis. Angew. Chem. Int. Ed. 58, 5327–5331 (2019).
Ge, S. L. et al. Bimetallic catalytic asymmetric tandem reaction of β-alkynyl ketones to synthesize 6,6-spiroketals. Angew. Chem. Int. Ed. 58, 4017–4021 (2019).
Xu, J. X. et al. Enantioselective vinylogous Michael-Adole reaction to synthesize spirocyclohexene pyrazolones in aqueous media. Org. Lett. 21, 1632–1636 (2019).
Acknowledgements
We appreciate the National Natural Science Foundation of China (Nos. 21871188 and 21890723) for financial support.
Author information
Authors and Affiliations
Contributions
X. H. performed the experiments. X. T. repeated data. X. Z. participated in the discussion. X. F. and L. L. supervised the project. X. F., L. L., and X. H. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Rui Shang and the other anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, X., Tang, X., Zhang, X. et al. Catalytic asymmetric Nakamura reaction by gold(I)/chiral N,Nʹ-dioxide-indium(III) or nickel(II) synergistic catalysis. Nat Commun 12, 3012 (2021). https://doi.org/10.1038/s41467-021-23105-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-23105-z
This article is cited by
-
Regioselective and asymmetric allylic alkylation of vinyl epoxides for the construction of allylic alcohols via synergistic catalysis
Science China Chemistry (2024)
-
Biomimetic approach to the catalytic enantioselective synthesis of tetracyclic isochroman
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.