Abstract
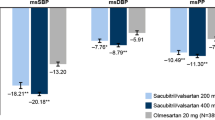
This randomized, double-blind phase 2 study assessed the efficacy and safety of sacubitril/allisartan, an angiotensin receptor neprilysin inhibitor, compared with placebo in Chinese patients with mild to moderate hypertension. Eligible patients aged 18–75 years (n = 235) with mild to moderate hypertension were randomized to receive sacubitril/allisartan 120 mg (n = 52), sacubitril/allisartan 240 mg (n = 52), sacubitril/allisartan 480 mg (n = 52), placebo (n = 26) or olmesartan 20 mg (n = 53) once daily for 8 weeks. The primary end point was a reduction in clinic systolic blood pressure from baseline with different doses of sacubitril/allisartan versus placebo at 8 weeks. Secondary efficacy variables included clinic diastolic blood pressure and 24-h ambulatory blood pressure for the comparison between sacubitril/allisartan and placebo at 8 weeks. Safety assessments included all adverse events and serious adverse events. Sacubitril/allisartan 480 mg/day provided a significantly greater reduction in clinic systolic blood pressure than placebo at 8 weeks (between-treatment difference: −9.1 mmHg [95% confidence interval −1.6 to −16.6 mmHg], P = 0.02). There were also significant reductions in 24-h, daytime and nighttime systolic and diastolic blood pressure for sacubitril/allisartan 480 mg/day compared with placebo (P ≤ 0.03). Similarly, a greater reduction in daytime systolic blood pressure was observed for sacubitril/allisartan 240 mg/day compared with placebo (between-treatment difference: −7.3 mmHg [95% confidence interval −0.5 to −14.0 mmHg], P = 0.04). Sacubitril/allisartan was well tolerated, and no cases of angioedema were reported. Sacubitril/allisartan is effective for the treatment of hypertension in Chinese patients and is well tolerated.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785–802.
Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang ZG, et al. China Hypertension Survey Investigators. Status of hypertension in China: Results from the China Hypertension Survey, 2012-2015. Circulation. 2018;137:2344–56.
Chen X, Xu SK, Guo QH, Hu Z, Wang HY, Yu J, et al. Barriers to blood pressure control in China in a large opportunistic screening. J Clin Hypertens. 2020;22:835–41.
Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind placebo-controlled, active comparator study. Lancet. 2010;375:1255–66.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004.
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20.
Wang TD, Chiang CE, Chao TH, Cheng HM, Wu YW, Wu YJ, et al. 2022 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. Acta Cardiol Sin. 2022;38:225–325.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee of clinical practice guidelines. Circulation. 2022;145:e895–1032.
Li Y, Li XH, Huang ZJ, Yang GP, Zhang GG, Zhao SP, et al. A randomized, double blind, placebo-controlled, multicenter phase II trial of Allisartan Isoproxil in essential hypertensive population at low-medium risk. PLoS One. 2015;10:e0117560.
Sun JC, Xu WJ, Hua HJ, Xiao Y, Chen XY, Gao ZW, et al. Pharmacodynamic and pharmacokinetic effects of S086, a novel angiotensin receptor neprilysin inhibitor. Biomed Pharmacother. 2020;19:110410.
Hu Y, Zhang H, Li XJ, Mai JJ, Yang LZ, Yan J, et al. A randomized, double-blind, placebo-controlled, single, and multiple dose-escalation Phase I clinical trial to investigate the safety, pharmacokinetic, and pharmacodynamic profiles of oral S086, a novel angiotensin receptor-neprilysin inhibitor, in healthy Chinese volunteers. Expert Opin Investig Drugs. 2022;31:977–85.
Huo Y, Li WM, Webb R, Zhao L, Wang Q, Guo WN. Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: A randomized, double-blind, 8-week study. J Clin Hypertens (Greenwich). 2019;21:67–76.
Rakugi H, Kario K, Yamaguchi M, Sasajima T, Gotou H, Zhang J. Efficacy of sacubitril/valsartan versus olmesartan in Japanese patients with essential hypertension: A randomized, double-blind, multicenter study. Hypertens Res. 2022;45:824–33.
Wang JG, Yukisada K, Sibulo A Jr, Hafeez K, Jia Y, Zhang J. Efficacy and safety of sacubitril/valsartan (LCZ696) add-on to amlodipine in Asian patients with systolic hypertension uncontrolled with amlodipine monotherapy. J Hypertens. 2017;35:877–85.
Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34:886–93c.
Campese VM, Lasseter KC, Ferrario CM, Smith WB, Ruddy MC, Grim CE, et al. Omapatrilat versus lisinopril: efficacy and neurohormonal profile in salt-sensitive hypertensive patients. Hypertension. 2001;38:1342–8.
Hubers SA, Brown NJ. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation. 2016;133:1115–24.
Kario K, Sun N, Chiang FT, Supasyndh O, Baek SH, Inubushi-Molessa A. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63:698–705.
Yano Y, Tanner RM, Sakhuja S, Jaeger BC, Booth JN 3rd, Abdalla M, et al. Association of daytime and nighttime blood pressure with cardiovascular disease events among African American individuals. JAMA Cadiol. 2019;4:910–7.
Kario K, Hoshide S, Mizuno H, Kabutoya T, Nishizawa M, Yoshida T, et al. Nighttime blood pressure phenotype and cardiovascular prognosis: Practitioner-based nationwide JAMP study. Circulation. 2020;142:1810–20.
Li Y, Wang JG. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension. 2013;61:278–83.
Sun NL, Jiang YN, Wang HY, Yuan YF, Cheng WL, Han QH, et al. Survey on sodium and potassium intake in patients with hypertension in China. J Clin Hypertens (Greenwich). 2021;23:1957–64.
Du SF, Wang HJ, Zhang B, Popkin BM. Dietary potassium intake remains low and sodium intake remains high, and most sodium is derived from home food preparation for Chinese adults, 1991-2015 trends. J Nutr. 2020;150:1230–9.
Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49:419–26.
Acknowledgements
We gratefully acknowledge all investigators at the participating centers and all patients for their commitment to the study.
Funding
The study was funded by Salubris Pharma (Shenzhen, Guangdong Province, China). The study investigators were also financially supported by grants from the National Natural Science Foundation of China (91639203 and 82070435), and Ministry of Science and Technology (grants 2018YFC1704902 and 2022YFC3601302), Beijing, China, from the Shanghai Municipal Commissions of Science and Technology (grant 19DZ2340200), and Health (a special grant for “leading academics”), and The Three-year Action Program of Shanghai Municipality for Strengthening the Construction of Public Health System (GWV-10.1-XK05) Big Data and Artificial Intelligence Application, Shanghai, China, and from The Clinical Research Program, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (grant 2018CR010), Shanghai, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JGW reports receiving lecture and consulting fees from Novartis, Omron, Servier, and Viatris; The other authors declared no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Zhang, W., Yan, J. et al. Efficacy and safety of sacubitril/allisartan for the treatment of primary hypertension: a phase 2 randomized, double-blind study. Hypertens Res 46, 2024–2032 (2023). https://doi.org/10.1038/s41440-023-01326-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01326-7