Abstract
The efficacy of renal denervation in the treatment of resistant hypertension has been controversial, and new strategies for its therapy are urgently needed. We performed the celiac ganglia neurolysis (CGN) or sham surgery on both spontaneously hypertensive rat (SHR) and Dahl salt-sensitive rat models of hypertension. Following CGN surgery in both strains, systolic blood pressure, diastolic blood pressure and mean arterial pressure were all lower than the levels in the respective sham surgery rats, which were maintained until the end of the study, 18 weeks postoperatively in SHRs and 12 weeks postoperatively in Dahl rats. CGN therapy destroyed ganglion cell structure and significantly inhibited celiac ganglia nerve viability. Four and twelve weeks after CGN, the plasma renin, angiotensin II and aldosterone levels were markedly attenuated, and the nitric oxide content was significantly increased in the CGN group compared with the respective sham surgery rats. However, CGN did not result in statistical difference in malondialdehyde levels compared with sham surgery in both strains. The CGN has efficacy in reducing high blood pressure and may be an alternative for resistant hypertension. Minimally invasive endoscopic ultrasound-guided celiac ganglia neurolysis (EUS-CGN) and percutaneous CGN are safe and convenient treatment approaches. Moreover, for hypertensive patients who need surgery due to abdominal disease or pain relief from pancreatic cancer, intraoperative CGN or EUS-CGN will be a good choice for hypertension therapy.

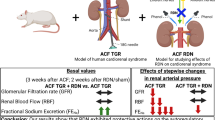
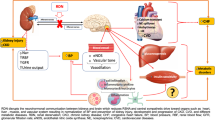
The graphical abstract of antihypertensive effect of CGN.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–94.
Li P, Liu B, Wu X, Lu Y, Qiu M, Shen Y, et al. Perirenal adipose afferent nerves sustain pathological high blood pressure in rats. Nat Commun. 2022;13:3130.
Mongeau JG. Pathogenesis of the essential hypertensions. Pediatr Nephrol. 1991;5:404–11.
Patel HP, Mitsnefes M. Advances in the pathogenesis and management of hypertensive crisis. Curr Opin Pediatr. 2005;17:210–4.
Yang P, Zhou L, Chen M, Zeng L, Ouyang Y, Zheng X, et al. Supplementation of amino acids and organic acids prevents the increase in blood pressure induced by high salt in Dahl salt-sensitive rats. Food Funct. 2022;13:891–903.
Polhemus DJ, Gao J, Scarborough AL, Trivedi R, McDonough KH, Goodchild TT, et al. Radiofrequency Renal Denervation Protects the Ischemic Heart via Inhibition of GRK2 and Increased Nitric Oxide Signaling. Circ Res. 2016;119:470–80.
Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: Durability of blood pressure reduction out to 24 months. Hypertension 2011;57:911–7.
Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet 2010;376:1903–9.
Roleder T, Skowerski M, Wiecek A, Adamczak M, Czerwienska B, Wanha W, et al. Long-term follow-up of renal arteries after radio-frequency catheter-based denervation using optical coherence tomography and angiography. Int J Cardiovasc Imaging. 2016;32:855–62.
Liang B, Zhao YX, Gu N. Renal denervation for resistant hypertension: Where do we stand? Curr Hypertens Rep. 2020;22:83.
Volz S, Spaak J, Elf J, Jagren C, Lundin C, Stenborg A, et al. Renal sympathetic denervation in Sweden: A report from the Swedish registry for renal denervation. J Hypertens. 2018;36:151–8.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401.
Desch S, Okon T, Heinemann D, Kulle K, Rohnert K, Sonnabend M, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension 2015;65:1202–8.
Mathiassen ON, Vase H, Bech JN, Christensen KL, Buus NH, Schroeder AP, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded 24-h blood pressure-based trial. J Hypertens. 2016;34:1639–47.
Schmieder RE, Ott C, Toennes SW, Bramlage P, Gertner M, Dawood O, et al. Phase II randomized sham-controlled study of renal denervation for individuals with uncontrolled hypertension - WAVE IV. J Hypertens. 2018;36:680–9.
Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): A randomised, sham-controlled, proof-of-concept trial. Lancet 2017;390:2160–70.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018;391:2335–45.
Fengler K, Rommel KP, Blazek S, Besler C, Hartung P, von Roeder M, et al. A three-arm randomized trial of different renal denervation devices and techniques in patients with resistant hypertension (RADIOSOUND-HTN). Circulation 2019;139:590–600.
Weber MA, Kirtane AJ, Weir MR, Radhakrishnan J, Das T, Berk M, et al. The REDUCE HTN: REINFORCE: Randomized, Sham-Controlled Trial of Bipolar Radiofrequency Renal Denervation for the Treatment of Hypertension. JACC Cardiovasc Inter. 2020;13:461–70.
Barbato E, Azizi M, Schmieder RE, Lauder L, Bohm M, Brouwers S, et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2023(e-pub ahead of print 2023/02/16; https://doi.org/10.1093/eurheartj/ehad054).
Crile G. The clinical results of celiac ganglionectomy in the treatment of essential hypertension. Ann Surg. 1938;107:909–16.
Asirvatham-Jeyaraj N, Gauthier MM, Banek CT, Ramesh A, Garver H, Fink GD, et al. Renal denervation and celiac ganglionectomy decrease mean arterial pressure similarly in genetically hypertensive Schlager (BPH/2J) Mice. Hypertension 2021;77:519–28.
Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci. 2010;154:66–73.
King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 2007;50:547–56.
Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 2013;61:806–11.
Ascunce G, Ribeiro A, Reis I, Rocha-Lima C, Sleeman D, Merchan J, et al. EUS visualization and direct celiac ganglia neurolysis predicts better pain relief in patients with pancreatic malignancy (with video). Gastrointest Endosc. 2011;73:267–74.
Kappelle WFW, Bleys R, van Wijck AJM, Siersema PD, Vleggaar FP. EUS-guided celiac ganglia neurolysis: a clinical and human cadaver study (with video). Gastrointest Endosc. 2017;86:655–63.
Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy 2013;45:362–9.
Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc. 2003;57:923–30.
Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–34.
Michaels AJ, Draganov PV. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World J Gastroenterol. 2007;13:3575–80.
Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–7.
Diener MK, Mihaljevic AL, Strobel O, Loos M, Schmidt T, Schneider M, et al. Periarterial divestment in pancreatic cancer surgery. Surgery 2021;169:1019–25.
Cai B, Lu Z, Neoptolemos JP, Diener MK, Li M, Yin L, et al. Sub-adventitial divestment technique for resecting artery-involved pancreatic cancer: a retrospective cohort study. Langenbecks Arch Surg. 2021;406:691–701.
Smithwick RH. Hypertensive vascular disease; results of and indications for splanchnicectomy. J Chronic Dis. 1955;1:477–96.
Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, et al. Sympathetic nerve activity in end-stage renal disease. Circulation 2002;106:1974–9.
Lee SH, Lim DH, Lee JH, Chang K, Koo JM, Park HJ. Long-term blood pressure control effect of celiac plexus block with Botulinum toxin. Toxins (Basel). 2016;8:51.
Lee CJ, Kim BK, Yoon KB, Lee HY, Dominiczak AF, Touyz RM, et al. Case of refractory hypertension controlled by repeated renal denervation and celiac Plexus block: A case of refractory sympathetic overload. Hypertension 2017;69:978–84.
Maeda S, Kuwahara-Otani S, Tanaka K, Hayakawa T, Seki M. Origin of efferent fibers of the renal plexus in the rat autonomic nervous system. J Vet Med Sci. 2014;76:763–5.
Chevendra V, Weaver LC. Distribution of splenic, mesenteric and renal neurons in sympathetic ganglia in rats. J Auton Nerv Syst. 1991;33:47–53.
Sripairojthikoon W, Wyss JM. Cells of origin of the sympathetic renal innervation in rat. Am J Physiol. 1987;252:F957–F963.
Gattone VH 2nd, Marfurt CF, Dallie S. Extrinsic innervation of the rat kidney: A retrograde tracing study. Am J Physiol. 1986;250:F189–96.
Lu H, Cassis LA, Kooi CW, Daugherty A. Structure and functions of angiotensinogen. Hypertens Res. 2016;39:492–500.
Streatfeild-James RM, Williamson D, Pike RN, Tewksbury D, Carrell RW, Coughlin PB. Angiotensinogen cleavage by renin: Importance of a structurally constrained N-terminus. FEBS Lett. 1998;436:267–70.
Borghi F, Seva-Pessoa B, Grassi-Kassisse DM. The adipose tissue and the involvement of the renin-angiotensin-aldosterone system in cardiometabolic syndrome. Cell Tissue Res. 2016;366:543–8.
Kanaide H, Ichiki T, Nishimura J, Hirano K. Cellular mechanism of vasoconstriction induced by angiotensin II: It remains to be determined. Circ Res. 2003;93:1015–7.
Bruneval P, Hinglais N, Alhenc-Gelas F, Tricottet V, Corvol P, Menard J, et al. Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization Histochem. 1986;85:73–80.
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7.
Kubota E, Dean RG, Hubner RA, Casley DJ, Johnston CI, Burrell LM. Differential tissue and enzyme inhibitory effects of the vasopeptidase inhibitor omapatrilat in the rat. Clin Sci (Lond). 2003;105:339–45.
Shorning BY, Jarde T, McCarthy A, Ashworth A, de Leng WW, Offerhaus GJ, et al. Intestinal renin-angiotensin system is stimulated after deletion of Lkb1. Gut 2012;61:202–13.
Ewert S, Spak E, Olbers T, Johnsson E, Edebo A, Fandriks L. Angiotensin II induced contraction of rat and human small intestinal wall musculature in vitro. Acta Physiol (Oxf). 2006;188:33–40.
Spak E, Casselbrant A, Olbers T, Lonroth H, Fandriks L. Angiotensin II-induced contractions in human jejunal wall musculature in vitro. Acta Physiol (Oxf). 2008;193:181–90.
Wong TP, Debnam ES, Leung PS. Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J Physiol. 2007;584:613–23.
Nagata S, Kato J, Kuwasako K, Kitamura K. Plasma and tissue levels of proangiotensin-12 and components of the renin-angiotensin system (RAS) following low- or high-salt feeding in rats. Peptides 2010;31:889–92.
Hamilton CA, Miller WH, Al-Benna S, Brosnan MJ, Drummond RD, McBride MW, et al. Strategies to reduce oxidative stress in cardiovascular disease. Clin Sci (Lond). 2004;106:219–34.
Liang M, Knox FG. Production and functional roles of nitric oxide in the proximal tubule. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1117–24.
Yoon Y, Song J, Hong SH, Kim JQ. Plasma nitric oxide concentrations and nitric oxide synthase gene polymorphisms in coronary artery disease. Clin Chem. 2000;46:1626–30.
Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4.
Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005;310:314–7.
Crassous PA, Couloubaly S, Huang C, Zhou Z, Baskaran P, Kim DD, et al. Soluble guanylyl cyclase is a target of angiotensin II-induced nitrosative stress in a hypertensive rat model. Am J Physiol Heart Circ Physiol. 2012;303:H597–H604.
Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation 2001;104:2338–43.
Galougahi KK, Liu CC, Gentile C, Kok C, Nunez A, Garcia A, et al. Glutathionylation mediates angiotensin II-induced eNOS uncoupling, amplifying NADPH oxidase-dependent endothelial dysfunction. J Am Heart Assoc. 2014;3:e000731.
Yamamoto E, Tamamaki N, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, et al. Excess salt causes cerebral neuronal apoptosis and inflammation in stroke-prone hypertensive rats through angiotensin II-induced NADPH oxidase activation. Stroke 2008;39:3049–56.
Lee DY, Wauquier F, Eid AA, Roman LJ, Ghosh-Choudhury G, Khazim K, et al. Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: role of mitochondrial reactive oxygen species. J Biol Chem. 2013;288:28668–86.
Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, et al. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab. 2012;15:201–8.
Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28.
Funding
This work was supported by the grant from the National Natural Science Foundation of China (81672449), Construction Program of Jiangsu Provincial Clinical Research Center Support System (BL2014084), the Project of Invigorating Health Care through Science, Technology and Education, Jiangsu Provincial Medical Outstanding Talent (to YM, JCRCA2016009), and Innovation Capability Development Project of Jiangsu Province (No. BM2015004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, S., Zhao, L., Wang, G. et al. Celiac ganglia neurolysis suppresses high blood pressure in rats. Hypertens Res 46, 1771–1781 (2023). https://doi.org/10.1038/s41440-023-01305-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01305-y
Keywords
This article is cited by
-
CT-Guided Percutaneous Renal Denervation with an Ozone–Oxygen Mixture Gas in Treating Resistant Hypertension
CardioVascular and Interventional Radiology (2024)
-
Celiac ganglia: potential new targets in neuromodulation for hypertension
Hypertension Research (2023)