Abstract
Glaucoma is the leading cause of preventable sight loss in the United Kingdom and the provision of timely glaucoma care has been highlighted as a significant challenge in recent years. Following a recent high-profile investigation, The Healthcare Safety Investigation Branch recommended the validation of risk stratification models to safeguard the vision-related quality of life of glaucoma patients. There continues to be no nationally agreed evidence-based risk stratification model for glaucoma care across the United Kingdom. Some models have used simple measures of disease staging such as visual field mean deviation as surrogates for risk, but more refined, individualised risk stratification models should include factors related to both visual impairment and visual disability. Candidate tools should also incorporate both ocular and systemic co-morbidities, rate of disease progression, visual needs and driving status and undergo clinical refinement and validation to justify implementation. The disruption to routine glaucoma care caused by the COVID-19 pandemic has only highlighted the importance of such risk stratification models and has accelerated their development, application and evaluation. This review aims to critically appraise the available evidence underpinning current approaches for glaucoma risk stratification and to discuss how these may be applied to contemporary glaucoma care within the United Kingdom. Further research will be essential to justify and validate the utility of glaucoma risk stratification models in everyday clinical practice.
摘要
青光眼在英国是可预防性视力丧失的主要原因之一。近年来, 提供及时的青光眼的诊疗已是一项重大挑战。在最近一项备受关注的调查之后, 医疗安全调查部门建议验证风险分层模型, 以保障青光眼患者与视力相关的生活质量。
在英国, 仍然没有基于循证医学证据的全国性青光眼诊疗风险的分层模型。一些模型使用简单的疾病分期, 如视野的平均偏差作为风险的预测因素, 但更精细的、个性化的风险分层模型应该包括与视力损害和视觉障碍相关的因素。预测因素还应包括引起眼部并发症的全身系统性疾病、疾病进展率、视力需求和驾驶状态, 并经过临床改进和验证以证明实施的合理性。新型冠状病毒肺炎 (COVID-19) 的大流行对常规青光眼的视力损伤证明了这种风险分层模型的重要性, 并加速了其开发、应用和评估。
本综述旨在批判性地评估当前青光眼风险分层方法的现有证据, 并讨论如何将这些方法应用于英国当代青光眼的诊疗中。进一步的研究将证明和验证青光眼风险分层模型在日常临床实践中的实用性。
Similar content being viewed by others
Introduction
Glaucoma is the leading cause of preventable sight loss in the United Kingdom. The provision of timely care has proven challenging in recent years, with a British Ophthalmological Surveillance Unit report published in 2017 showing that over twenty people per month suffered permanent and severe vision loss as a consequence of delayed follow-up [1]. In response to this, a formal investigation by the Healthcare Safety Investigation Branch recommended several measures including the validation of risk stratification models in order to safeguard the vision-related quality of life of glaucoma patients [2].
Currently there continues to be no nationally agreed, evidence-based risk stratification model for glaucoma. Some have used simple measures of disease staging such as visual field mean deviation as surrogates for risk, but more refined, individualised risk stratification models should consider factors related to both visual impairment and disability in patients with glaucoma. Candidate tools should incorporate both ocular and systemic co-morbidities, rate of disease progression, visual needs and driving status and undergo clinical refinement and validation. The disruption to routine glaucoma care caused by the COVID-19 pandemic [3] has highlighted the importance of risk stratification models and has accelerated their development and application.
This review aims to critically appraise the available evidence underpinning current approaches for risk stratification and to examine how these may or may not be applied to global contemporary glaucoma care. We aim to suggest areas of further research that are essential to justify and validate the utility of glaucoma risk stratification models in everyday clinical practice.
What is risk stratification?
The term risk stratification is often used synonymously with prognostic and predictive modelling and is gaining increased importance within the NHS and around the world, partly in response to a greater demand on services as well as the increased availability of data and analysis tools that have made new models of care possible. In the UK, an estimated one million annual hospital visits take place for glaucoma and as many as forty-two percent of glaucoma patients suffer preventable vision loss due to delays in treatment [4]. It is therefore essential to allocate resources efficiently in order to manage the burden of disease, avoid overtreatment and minimise the risk of adverse outcomes.
Prognostic modelling
The process of determining a prognosis is a form of forecasting, with parallels in economics and meteorology, and involves estimation of the “probability or risk of an individual developing a particular state of health / outcome over a specific time, based on their clinical and non-clinical profile” [5]. This can be useful for ‘case finding’ of those at risk of a particular condition who can then be stratified according to need. Some may require early surgery, while others can be managed with observation or eye drops alone.
The prognosis will determine the most appropriate setting and frequency of follow up for each individual, ranging from infrequent visits in the community for suspects and those at low risk of change through to close surveillance within a shared care or hospital setting for those with advanced disease or at risk of rapid progression. Of particular importance is the avoidance of ‘triple fail’ outcomes that are simultaneously high cost, low quality and represent a poor patient experience [6]. Registration of an individual as severely sight impaired would be an example of such an outcome.
Prognostication is also important in health planning to understand the ongoing and future needs of the population. When applied to a population, it is roughly analogous to screening and arguably should have similar pre-requisites based upon the World Health Organisation (WHO) Wilson and Jungner criteria [7]. The condition should be important, the natural history understood, with an early latent stage identifiable through an acceptable and accurate test, and modifiable with a cost-effective treatment and an agreed policy on whom to treat. With regards to glaucoma however, there remain important uncertainties in all of these areas.
In research, prognostic studies are conventionally divided into development, validation and impact studies. Development studies use multivariable models to identify important predictors and assign weights to each. They differ from aetiology studies in so far as they are interested in the combined effect of various risk factors rather than the relative contribution of an individual predictor with and without adjustment for confounders. They are commonly calibrated using internal validation techniques or tested against other populations in formal validation studies. The overall effect of the application of these models on decision making and patient outcomes is then assessed in impact studies [5].
Different types of prognostic model
Most models are based either on clinical judgment, thresholds, or multivariable predictions [8]. Clinical judgment is the most intuitive but is limited by cognitive biases and difficulty in scaling individual interventions to the wider population. A notable example is the difficulty amongst ophthalmologists in predicting the risk of conversion to glaucoma from ocular hypertension [9].
Threshold and predictive analyses are analogous to event and trend-based analyses routinely used to determine progression of visual fields. Threshold models are simpler and aim to ‘catch all’ individuals who meet predefined criteria but are less likely to detect rapid change and are susceptible to regression to the mean where extreme events will tend to self-correct when measured subsequently even in the absence of an intervention [10]. This is a key limitation of studies where drops are switched at high intraocular pressures (IOP) since subsequent measurements will tend to be lower, whether or not the drops have been changed.
Despite this, thresholds offer a simple, reproducible and transparent way to efficiently sort cross-sectional data and have been useful in service planning and prioritisation during the pandemic. Bommakanti et al. described the successful application of a scoring system that offset high risk features for COVID-19 such as age and medical co-morbidities against those for glaucoma including IOP > 30 mmHg, recent surgery, extensive visual field loss and monocularity [11].
Predictive models offer the most promise and use multiple variable regression or Artificial Intelligence (AI) techniques to make predictions of future risk. However, these are more complex to apply and are limited by the generalisability of the datasets upon which they were developed as well as the impact of the population within which they are to be applied. ‘Impactability’ encompasses the idea that not all individuals will have risks that can be mitigated equally and this can be modelled separately with interventions offered to those that are more likely to respond, to benefit or to have correctable gaps in their care [12]. This can raise ethical questions as treatment is offered according to efficiency rather than need.
Within this context, it is easy to see the limitations of the original National Institute of Clinical Excellence (NICE) guidance on the treatment of glaucoma [13]. While it appeared to be based on the validated predictive models derived from the Ocular Hypertension Treatment Study [14] (OHTS), it was applied inconsistently within the community as a simple threshold with poor diagnostic utility.
Much is made of the potential of AI to transform care but previous audits of visual field and imaging datasets have shown that useful tools to examine hospital databases and identify those that are high risk or changing rapidly already exist [15]. Electronic Record Systems such as OpenEyes and Medisoft have a wide array of search and audit functions that offer powerful tools to examine patient groups. These range from complex statistical analysis to simple text string searches for high risk terms such as ‘only eye’. Engagement with industry is necessary to ensure that the outputs from these systems are fully exportable and the software interfaces used to manipulate and examine this data are intuitive, easy to learn and simple to use.
At risk of what? - Glaucoma and sight loss
Glaucoma is frequently described as the leading cause of irreversible sight loss but encompasses a broad spectrum of disease. Patients with glaucoma prioritise central visual acuity and mobility [16] and fear of blindness is a common concern expressed by over half of new patients following diagnosis [17]. Retrospective evaluations of deceased patients have found a mean survival of less than 10 years following diagnosis [18,19,20] with approximately 10% becoming severely sight impaired before death [21]. Approximately 10% of severe sight impairment certifications within the UK and Europe are due to glaucoma [22, 23] with 11 patients per 1000 converting to blindness each year [24]. As would be expected, prospective cohorts show lower rates of sight loss and a recent report from the UK found a 5.5% risk of blindness over 20 years with a median time to death of 16 years [25]. Within this cohort, a third of eyes did not progress, a third progressed up to two visual field ‘grades’ and a third progressed by more.
At risk of what? - Glaucoma and disability
Those with less advanced disease may struggle with individual tasks but how far these correspond to functional measures and the patient’s own priorities and insight varies. Difficulty with reading is a common complaint. Visual field defects make scanning and searching text more difficult and reduce the number the letters that can be read at each fixation, slowing reading speeds [26] and making reading harder [27]. Field defects also lead to poor balance, a higher incidence of falls and fractures and with a greater fear of falling leading to a reduction in physical activity [28]. Those with glaucoma are more likely to cease driving or at least modify their driving behaviour by making more saccadic movements and avoiding unfavourable conditions, such as driving at night and in unfamiliar environments [29]. As a result, data on vehicle collisions is mixed but it is known that visual field defects (and not reduced visual acuity) are associated with an increased risk of motor vehicle accidents [30]. The combined effect of these functional impairments and loss of freedoms is that individuals with glaucoma are ten to twelve times more likely to suffer from anxiety and depression when compared to aged matched controls, even after adjusting for other comorbidities [31].
The natural history of Glaucoma
Patients can develop blindness due to disease progression despite treatment, late diagnosis or late presentation and because of co-morbid disease [21]. In some cases, such as retinal vascular occlusions [32] or cataract [33], this co-morbidity is directly related to their glaucoma or its management whilst in others, such as age-related macular degeneration, it is confounded by age and frailty.
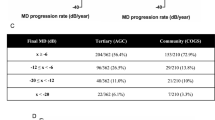
The importance and difficulty in differentiating between those who will and will not progress is apparent in some of the earliest modern studies [34]. Seminal studies have shown an overall benefit from pressure lowering treatments, but a wide variation in responses to intervention. Within the control arm of the Early Manifest Glaucoma Trial (EMGT), the median time to progress from a normal field to blindness was predicted to be 70 years, suggesting that even without treatment, most patients with glaucoma will not go blind during their lifetime. However, this falsely assumes that deterioration is linear and the same study also found higher rates of visual field progression in the older section of their cohort [35]. This was most likely due to late decompensation, since structural changes were more commonly seen in fellow eyes without field loss than in those with established disease [36]. Other studies have shown that imaging is more useful before field loss has occurred while perimetry is more useful after [37]. Within the EMGT, the median times for visual field progression were 19.5 months in Pseudoexfoliative Glaucoma, 44.8 months in Primary Open Angle Glaucoma, and 61.1 months in Normal Tension Glaucoma (NTG), with very little visual field progression in those under 68 years old. A large UK hospital-based study of over 4000 eyes with 5 or more visual fields found that 21% of individuals progressed at a rate >0.5 dB/year and 2% progressed more rapidly than 2 dB/year. The median rate of progression for the whole cohort was 0.1 dB/year [15]. Older age, higher peak IOP, worse baseline damage, pseudoexfoliative glaucoma and cardiovascular disease are known to be associated with more rapid progression [38] but there is broad overlap between groups and the importance of identifying the small proportion of rapid progressors over a shorter assessment period needs to be balanced against the resource needs of the larger proportion of slow-moderate progressors who need longer follow up to confirm deterioration. Structural retinal nerve fibre layer (RNFL) change of ≥5 μm is often considered as clinically relevant as it exceeds the test-retest variability commonly seen with most OCT devices, though again, this threshold approach appears less sensitive than trend-based analysis [39].
Those with progressive disease have a worse life expectancy often in spite of good pressure control [40] and for some individuals sight loss may even be an inevitable pre-morbid event. Modern treatments and approaches have slowed rates of progression [41] but increases in life expectancy and demographic shifts make prevention of vision loss increasingly harder to achieve.
Which Glaucoma patients deteriorate?
There are over a hundred prognostic risk factors that have been linked to visual field progression in glaucoma [42]. The OHTS study found that older age, higher IOP, thinner central corneal thickness (CCT), larger vertical cup-to-disc ratio (CDR) and increased visual field pattern standard deviation were predictors of conversion to glaucoma [14]. In the Collaborative Normal Tension Glaucoma Study (CNGTS) female gender, black ethnicity, migraine/Raynaud’s disease and disc haemorrhages were associated with visual field progression [43]. However this conflicted with a subsequent a systematic review which suggested gender and Raynaud’s disease were unlikely to be associated with progression. It found that older age and disc haemorrhages were clearly associated and baseline field loss and IOP, pseudoexfoliation, thinner CCT and peri-papillary atrophy (in NTG) were likely associated with subsequent visual field loss [42].
In Angle Closure Disease, both iridotomies [44] and lens extraction [45] are known to be less effective following the onset of glaucomatous optic neuropathy, suggesting that once the trabecular meshwork has been compromised, ongoing monitoring becomes necessary. Similarly, in pigment dispersion syndrome, iridotomy may be helpful in some patients with early disease [46] but is less effective once the IOP is raised [47]. Uveitic and other secondary glaucomas can have a variable and often aggressive course with rapid progression to sight loss either from the underlying pathology and its treatment, or subsequent severe and often high pressures refractory to treatment.
Iatrogenic risks
Care should be taken to avoid unnecessary treatment, in order to minimise cost, harm, anxiety and other iatrogenic effects. The incidence of ocular surface disease doubles after the commencement of drops [48] and surgery understandably leads to a more intense requirement for face-to-face clinical review and is also associated with risks including potential sight loss. The most recent survey of U.K. trabeculectomy outcomes reported 80% unqualified success at 2 years (IOP ≤ 21 with ≥20% reduction in IOP) but highlighted the need for increased follow up intensity and the need for post-operative interventions. Following filtration surgery, 43% required suture manipulations, 27% required subconjunctival injections, 31% went on to require cataract surgery, 16% received bleb needling procedures and 7% underwent revision for hypotony. Sight threatening complications were less common, with endophthalmitis seen in 1% of eyes and a drop in vision (more than two Snellen lines) in 6% of cases [49]. Minimally invasive surgery appears safer, but robust evidence of efficacy is lacking and where randomised studies have been performed, these devices only have a marginal advantage against standard care [50]. Direct care costs increase linearly with each stage of disease [51] and while this adds an economic incentive to the need to reduce progression, it should to be balanced against the risk of medicalising advanced age, for the vast majority of patients who are unlikely to develop functionally relevant sight loss within their lifetime.
Implementation of risk stratification in the United Kingdom
There has always been debate over how to apply risk stratification to patients and how best to structure the service. Over 50 years ago, Hollows and Graham advocated “measurement of facility of aqueous outflow to separate the ocular hypertensive sheep from the pre-glaucomatous goats” [34]. At that time, the water drinking test was in common use and after falling out of fashion for decades, it has attracted renewed interest as a marker of progression and predictor of response to treatment [52]. Attitudes to shared care have similarly varied over time. Modern schemes have been in place in the UK for over 30 years with various approaches to setting, structure and staffing [53].
There was revived interest in these schemes after the 2009 CG85 NICE guidance led to a sudden increase in referrals from community optometrists. In the four years preceding issuance of this guidance, there were thirteen instances of total loss of vision attributed to delays in follow up reported to the National Patient Safety Agency. This led them to issue guidance advising local organisation to audit their capacity and attendance rates, streamline their booking systems and disseminate the NICE guidance with special regards to appropriate follow up intervals for different patient groups [54].
More recently, following a high profile case of the sight loss in a young patient [55], the Healthcare Safety Investigation Branch (HSIB) undertook an examination of the lack of timely monitoring within glaucoma clinics in the UK. The case was noticeable both for the size of settlement (£3.2 million) and the combination of factors that led to the poor outcome. These included late presentation, young age and pregnancy along with delays in monitoring and treatment. They recommended that the “Royal College of Ophthalmologists (RCOphth) agree criteria for the risk stratification of patients with glaucoma so that practice can be standardised across the NHS” and suggested that the International Glaucoma Association (now known as Glaucoma UK) fund “research into the development and evaluation of an automated, predictive risk stratification tool” [2].
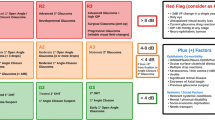
The RCOphth in conjunction with the UK & Éire Glaucoma Society [56] (The UK and Éire Glaucoma Society (UKEGS) is a non-profit national scientific society, and part of Glaucoma UK) as well as other organisations [57], have proposed Red / Amber / Green risk stratification tools that divide glaucoma into groups based primarily on visual field mean deviation (MD) (Fig. 1) as a response to the need for risk stratification during the recovery period following the COVID-19 pandemic. The RCOphth/UKEGS guidance uses the Glauc-Strat-Fast protocol (Fig. 1a) which was developed in Birmingham without formal clinical validation. The system has three broad diagnostic categories, each subdivided into three further strata, with nine groups in total, modifiable by ‘red flags’ and ‘plus factors’. Progressive glaucoma is listed as a single diagnosis within the top group and also as a ‘red flag’ modifier. Within the other eight groups, disease severity is synonymous with risk. The lack of a graded approach to progression is a limitation, since it is the interaction of the rate of progression and disease severity that commonly determine disability. As illustrated by Caprioli [58] (Fig. 2), early intervention can have a dramatic effect on the course of disease, whilst even in advanced disease, progression can sometimes be halted if the IOP is well controlled [59].
(Modified from Caprioli, 2008) [58].
The protocol also appears more complex and less modifiable than other systems which simply describe diagnostic categories and group them into low, medium and high risk, with inclusion and exclusion criteria specified for each group. The latter approach is particularly useful since expertise, complexity, case mix and resource are likely to vary substantially between units. In addition, where specific phenotypes, such as ‘treated primary angle closure’, are included, both the diagnostic criteria and patient pathway can be outlined within the category description, removing the need to refer to additional appendices (Fig. 1b, c). Simplicity is also key, given the heavy administrative demands that already exist in hospital and community clinics. Systems which are simpler, more intuitive and easier to understand are more likely to be readily adopted.
Post-hoc validation of the Glauc-Strat-Fast protocol using longitudinal data from the LiGHT trial [60] did show a significant association between baseline stratification and the overall number of attendances, number of treatment escalations, the need for trabeculectomy and overall loss of visual field over three years of follow up, but did not find an association with the rate of visual field progression [61]. Further studies are required to validate the applicability of this protocol to more advanced glaucomas as well as its generalisability to routine clinical practice.
In all of these systems, there is a danger of overreliance on visual field metrics which will miss structural progression in early disease [37] and do not account for the type of defect. For many patients, a central scotoma with a small mean deviation can have a profound impact while conversely a large peripheral defect can be of little functional significance.
Special care should be taken in the development and application of this type of guidance. Guidelines are most useful where they offer robust scientific evidence to address gaps in knowledge but they often do injustice to the complexities of medicine. Flaws in their design and application can exacerbate problems and cause to harm patients, practitioners and systems [62] and the limitations of guidelines and the importance of local leadership are being increasingly recognised within NHS vanguard projects [63]. Controversies over guidelines for AMD and glaucoma have understandably left clinicians wary of their use and while few would doubt the importance of new tools in helping to redesign and rationalise services, their success will depend on how far they free clinicians and patients rather than constrain them.
Conclusion
Risk stratification is a form of prognostication or forecasting. The criteria used can be based on clinical judgement, thresholds or predictive models and these all need to be designed, tested and validated taking in to account the risk factors for disease, the aims of treatment, the impact of intervention and resource constraints. Even without treatment, most patients with glaucoma will not lose vision but around 10% will go blind due to late presentation or late treatment, progression despite treatment or comorbid disease. Red/Amber/Green threshold models that rely on visual field mean deviation as a functional indicator have been proposed as a stop-gap until practical predictive models can be designed and deployed. Each has its own benefits and limitations and their application should be adapted according to local need and resource with the support of further robust validation studies.
Summary
What is known about this topic
-
Glaucoma is the leading cause of preventable sight loss in the United Kingdom and the provision of timely glaucoma care is known to be a challenge.
-
There is no nationally agreed evidence-based risk stratification model for glaucoma care across the United Kingdom.
-
The disruption to care caused by the pandemic highlighted the importance of risk stratification models and has accelerated their development, application and evaluation.
What this review adds
-
Risk stratification is a form of prognostication or forecasting.
-
The criteria utilised need to be validated taking in to account risk factors for disease, the aims and impact of treatment and resource constraints.
-
Models that rely on visual field mean deviation as a functional indicator have been proposed as an interim solution until practical predictive models are designed and deployed.
-
Benefits and limitations exist with all approaches and their application should be tailored to local need and resource and justified with further robust validation studies.
References
Foot B, MacEwen C. Surveillance of sight loss due to delay in ophthalmic treatment or review: frequency, cause and outcome. Eye (Lond). 2017;31:771–5.
Lack of Timely Monitoring of Patients with Glaucoma. Healthcare Safety Investigation 2019/001. United Kingdom: Healthcare Safety Investigation Branch; https://www.hsib.org.uk/investigations-and-reports/lack-of-timely-monitoring-of-patients-with-glaucoma/ 2020.
Jayaram H, Strouthidis NG, Gazzard G. The COVID-19 pandemic will redefine the future delivery of glaucoma care. Eye (Lond). 2020;34:1203–5.
Park I, Gale J, Skalicky SE. Health economic analysis in glaucoma. J Glaucoma. 2020;29:304–11.
Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? Bmj. 2009;338:b375.
Lewis G, Kirkham H, Duncan I, Vaithianathan R. How health systems could avert ‘triple fail’ events that are harmful, are costly, and result in poor patient satisfaction. Health Aff (Millwood). 2013;32:669–76.
Wilson JMG, Jungner G. Principles and practice of screening for disease/JMG Wilson, G Jungner. Geneva: World Health Organization; 1968.
Lewis G, Curry N, Bardsley M. Choosing a predictive risk model: a guide for commissioners in England. London: Nuffield Trust 2011;20.
Mansberger SL, Cioffi GA. The probability of glaucoma from ocular hypertension determined by ophthalmologists in comparison to a risk calculator. J Glaucoma. 2006;15:426–31.
Roland M, Abel G. Reducing emergency admissions: are we on the right track? Bmj. 2012;345:e6017.
Bommakanti NK, Zhou Y, Ehrlich JR, Elam AR, John D, Kamat SS, et al. Application of the sight outcomes research collaborative ophthalmology data repository for triaging patients with glaucoma and clinic appointments during pandemics such as COVID-19. JAMA Ophthalmol. 2020;138:974–80.
Lewis G. Next steps for risk stratification in the NHS. London: NHS England; 2015.
Glaucoma: diagnosis and management Clinical Guidelines. London: National Institute for Health and Care Excellence; https://pubmed.ncbi.nlm.nih.gov/29106798/ 2017.
Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20.
Kirwan JF, Hustler A, Bobat H, Toms L, Crabb DP, McNaught AI. Portsmouth visual field database: an audit of glaucoma progression. Eye (Lond). 2014;28:974–9.
Aspinall PA, Johnson ZK, Azuara-Blanco A, Montarzino A, Brice R, Vickers A. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1907–15.
Janz NK, Wren PA, Lichter PR, Musch DC, Gillespie BW, Guire KE. Quality of life in newly diagnosed glaucoma patients: the collaborative initial glaucoma treatment study. Ophthalmology. 2001;108:887–97.
Ang GS, Eke T. Lifetime visual prognosis for patients with primary open-angle glaucoma. Eye (Lond). 2007;21:604–8.
Ernest PJ, Busch MJ, Webers CA, Beckers HJ, Hendrikse F, Prins MH, et al. Prevalence of end-of-life visual impairment in patients followed for glaucoma. Acta Ophthalmol. 2013;91:738–43.
Forsman E, Kivelä T, Vesti E. Lifetime visual disability in open-angle glaucoma and ocular hypertension. J Glaucoma. 2007;16:313–9.
Mokhles P, Schouten JS, Beckers HJ, Azuara-Blanco A, Tuulonen A, Webers CA. Glaucoma blindness at the end of life. Acta Ophthalmol. 2017;95:10–11.
Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol. 2014;98:629–38.
Rahman F, Zekite A, Bunce C, Jayaram H, Flanagan D. Recent trends in vision impairment certifications in England and Wales. Eye (Lond). 2020;34:1271–8.
Rossetti L, Digiuni M, Montesano G, Centofanti M, Fea AM, Iester M, et al. Blindness and glaucoma: a multicenter data review from 7 academic eye clinics. PLoS One. 2015;10:e0136632.
King C, Sherwin JC, Ratnarajan G, Salmon JF. Twenty-year outcomes in patients with newly diagnosed glaucoma: mortality and visual function. Br J Ophthalmol. 2018;102:1663–6.
Kwon M, Liu R, Patel BN, Girkin C. Slow reading in glaucoma: is it due to the shrinking visual span in central vision? Invest Ophthalmol Vis Sci. 2017;58:5810–8.
Nguyen AM, van Landingham SW, Massof RW, Rubin GS, Ramulu PY. Reading ability and reading engagement in older adults with glaucoma. Invest Ophthalmol Vis Sci. 2014;55:5284–90.
Sotimehin AE, Ramulu PY. Measuring disability in glaucoma. J Glaucoma. 2018;27:939–49.
Blane A. Through the looking glass: a review of the literature investigating the impact of glaucoma on crash risk, driving performance, and driver self-regulation in older drivers. J Glaucoma. 2016;25:113–21.
Kotecha A, Spratt A, Viswanathan A. Visual function and fitness to drive. Br Med Bull. 2008;87:163–74.
Zhang X, Olson DJ, Le P, Lin FC, Fleischman D, Davis RM. The association between glaucoma, anxiety, and depression in a large population. Am J Ophthalmol. 2017;183:37–41.
Yin X, Li J, Zhang B, Lu P. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652–9.
Chang PY, Wang JY, Chang SW, Chang YC. Changes in ocular hypotensive drug usage for glaucoma treatment after cataract surgery: a nationwide population-based study in Taiwan. J Glaucoma. 2018;27:600–5.
Hollows FC, Graham PA. Intra-ocular pressure, glaucoma, and glaucoma suspects in a defined population. Br J Ophthalmol. 1966;50:570–86.
Heijl A, Bengtsson B, Hyman L, Leske MC. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–6.
Öhnell H, Heijl A, Brenner L, Anderson H, Bengtsson B. Structural and functional progression in the early manifest glaucoma trial. Ophthalmology. 2016;123:1173–80.
Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53:6939–46.
Kim JH, Rabiolo A, Morales E, Yu F, Afifi AA, Nouri-Mahdavi K, et al. Risk factors for fast visual field progression in glaucoma. Am J Ophthalmol. 2019;207:268–78.
Thompson AC, Jammal AA, Berchuck SI, Mariottoni EB, Wu Z, Daga FB, et al. Comparing the rule of 5 to trend-based analysis for detecting glaucoma progression on OCT. Ophthalmol Glaucoma. 2020;3:414–20.
Tattersall CL, Vernon SA, Negi A. Is poor life expectancy a predictive factor in the progression of primary open angle glaucoma? Eye (Lond). 2005;19:387–91.
Boodhna T, Saunders LJ, Crabb DP. Are rates of vision loss in patients in English glaucoma clinics slowing down over time? Trends from a decade of data. Eye (Lond). 2015;29:1613–9.
Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013;120:512–9.
Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708.
Nolan WP, Foster PJ, Devereux JG, Uranchimeg D, Johnson GJ, Baasanhu J. YAG laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol. 2000;84:1255–9.
Song MK, Sung KR, Shin JW, Jo YH, Won HJ. Glaucomatous progression after lens extraction in primary angle closure disease spectrum. J Glaucoma. 2020;29:711–7.
Gandolfi SA, Ungaro N, Tardini MG, Ghirardini S, Carta A, Mora P. A 10-year follow-up to determine the effect of YAG laser iridotomy on the natural history of pigment dispersion syndrome: a randomized clinical trial. JAMA Ophthalmol. 2014;132:1433–8.
Scott A, Kotecha A, Bunce C, Balidis M, Garway-Heath DF, Miller MH, et al. YAG laser peripheral iridotomy for the prevention of pigment dispersion glaucoma a prospective, randomized, controlled trial. Ophthalmology. 2011;118:468–73.
Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785–93.
Kirwan JF, Lockwood AJ, Shah P, Macleod A, Broadway DC, King AJ, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology. 2013;120:2532–9.
Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12:e0183142.
Traverso CE, Walt JG, Kelly SP, Hommer AH, Bron AM, Denis P, et al. Direct costs of glaucoma and severity of the disease: a multinational long term study of resource utilisation in Europe. Br J Ophthalmol. 2005;89:1245–9.
Susanna R Jr., Clement C, Goldberg I, Hatanaka M. Applications of the water drinking test in glaucoma management. Clin Exp Ophthalmol. 2017;45:625–31.
Vernon SA, Adair A. Shared care in glaucoma: a national study of secondary care lead schemes in England. Eye (Lond). 2010;24:265–9.
Preventing Delay to Follow Up for Patients With Glaucoma (NPSA/2009/RRR004). Rapid Response Report - From Reporting to Learning. London, United Kingdom: NHS National Patient Safety Agency; https://www.ahpo.net/assets/090609_rrr_glaucoma_final.pdf 2009.
Torjesen I. Glaucoma report: patients’ sight is put at risk by treatment delays. Bmj. 2020;368:m103.
Joint RCOphth and UKEGS Glaucoma Risk Stratification Tool: Royal College of Ophthalmologists; https://glaucoma.uk/wpcontent/uploads/2020/09/Glaucoma-Risk-Stratification-Tool-1.pdf 2020.
How to stratify the risk or complexity for glaucoma to direct patients to appropriate clinics: U.K. Ophthalmology Alliance; 2020:https://uk-oa.co.uk/wp-content/uploads/2020/2002/Glaucoma-Risk-and-Virtuals_PDF.pdf.
Caprioli J. The importance of rates in glaucoma. Am J Ophthalmol. 2008;145:191–2.
The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol. 2000;130:429–40.
Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393:1505–16.
Konstantakopoulou E, Kastner A, Gazzard G, Jayaram H. Validation of the RCOphth and UKEGS glaucoma risk stratification tool ‘GLAUC-STRAT-fast’. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2021-320968 2022.
Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. Bmj. 1999;318:527–30.
Iacobucci G. NHS long term plan: care to be shifted away from hospitals in “21st century” service model. Bmj. 2019;364:l85.
Acknowledgements
GG is employed by UCL and supported by grants from the National Institute for Health Research (HTA 09/104/40), Moorfields Eye Charity, British Council to Prevent Blindness, Fight For Sight and the International Glaucoma Association. HJ is supported by the Moorfields Eye Charity. GG and HJ are grateful for the support of the National Institute for Health Research Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and the UCL Institute of Ophthalmology. Views expressed in this paper are those of the authors and not necessarily those of any funding body or the UK Department of Health. This project was supported by a grant from the Moorfields Eye Charity (Reference: GR001214). GG is employed by UCL and supported by grants from the National Institute for Health Research (HTA 09/104/40), Moorfields Eye Charity, British Council to Prevent Blindness, Fight For Sight and the Glaucoma UK. HJ is supported by the Moorfields Eye Charity. GG and HJ are grateful for the support of the National Institute for Health Research Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and the UCL Institute of Ophthalmology. The views expressed in this paper are those of the authors and not necessarily those of any funding body or the UK Department of Health.
Author information
Authors and Affiliations
Contributions
AP, AK, EK, GG & HJ all developed the theme of this review, contributed to the drafts and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors developed the review structure and approach. AP, AK, HJ & GG contributed to the first draft and all authors approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poostchi, A., Kastner, A., Konstantakopoulou, E. et al. Clinical risk stratification in glaucoma. Eye 37, 3121–3127 (2023). https://doi.org/10.1038/s41433-023-02480-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02480-5