Abstract
Introduction
Serum eye drops (SED) are an important treatment for patients with chronic and severe ocular surface disease (OSD). Despite a long history of use, there is a paucity of information on patient-reported outcomes, particularly comparing autologous SED (Auto-SED) and allogeneic SED (Allo-SED). National Health Service Blood and Transplant is the national provider of SED service for patients in the UK.
Purpose
To evaluate and compare patient-reported outcome measures (PROMs) in patients receiving Auto-SED and Allo-SED for severe OSD.
Materials and methods
PROMs were retrospectively collected from all new patients commencing treatment with Auto-SED and Allo-SED between January 2017 and September 2018, using the Ocular Surface Disease Index (OSDI) 12-item questionnaire. A linear mixed model was used to evaluate the change in OSDI scores between baseline and follow-up.
Results
During the study period, 279 patients who received either Auto-SED (n = 71) or Allo-SED (n = 208) were included in the analysis. Baseline and follow-up OSDI scores were available for 161 of these (49 Auto-SED and 112 Allo-SED). There was a significant reduction in mean OSDI score for both Auto-SED (59.06–24.63, p < 0.001) and Allo-SED (64.21–34.37, p < 0.001). There was no significant difference between Auto-SED and Allo-SED patients in terms of the reduction in the OSDI score (p = 0.27).
Conclusion
Both Auto-SED and Allo-SED were associated with improvements in the quality of life of patients with chronic and severe OSD. Auto-SED and Allo-SED were equally effective in relieving the symptoms of OSD.
Similar content being viewed by others
Introduction
Ocular surface disease (OSD) is a group of disorders of diverse pathogenesis, in which disease results from the failure of mechanisms responsible for maintaining the homeostasis of a healthy ocular surface, including the tear film. This may occur in a number of conditions associated with a dry eye including immune-mediated diseases, such as in Sjögren’s syndrome and graft-versus-host disease (GVHD), and non-immune-related conditions such as neurotrophic keratopathy, meibomian gland disease, injury (physical, thermal or chemical) and other non-immune dry eye diseases [1]. Severe OSD can lead to complications including chronic inflammation, infection and scarring. The associated discomfort and sometimes pain can have a major impact on the patient’s quality of life [2]. First-line treatment options for OSD include ocular lubricants, gels and ointments, often combined with punctal plugs and or cautery, depending on the severity of the condition. If these treatments are ineffective, treatment with serum eye drops (SED) may be considered.
The clinical use of SED, for patients with OSD, specifically dry eye disease, was first reported in 1984 [3]. Since then, SED have been widely used to treat a variety of OSD with multiple underlying aetiologies, usually with little to no side effects and resulting in generally positive clinical outcomes [4]. The large majority of clinical reports relating to SED utilise autologous SED (Auto-SED), manufactured from the patient’s own blood [4].
The availability of allogeneic SED (Allo-SED) was made possible in the UK by National Health Service Blood and Transplant (NHSBT), who have the requisite experience, laboratory facilities and quality management systems for screening donors, collecting blood from healthy voluntary donors and manufacturing the SED, based on many years of providing an Auto-SED service. This had clear advantages for patients who were not medically suitable to donate their own blood, due to co-existing medical conditions such as anaemia, cardiovascular or neurological diseases or poor venous access. It also meant that for urgent requests SED would be immediately available ‘off the shelf’.
The first clinical use of Allo-SED was reported by Chiang et al. in 2007 [5], who used Allo-SED to treat two patients with GVHD. The authors reported that the treatment was safe and effective in both cases. The same group reported a larger case series in 2009 [6], demonstrating the safe and effective use of Allo-SED, with or without amniotic membrane transplantation, to treat 36 patients with persistent corneal epithelial defects (PEDs).
The New Zealand Blood Service (NZBS) provides both Auto-SED and Allo-SED. A retrospective crossover study, based on 27 patients who had been initially provided with Auto-SED by the NZBS but subsequently switched to Allo-SED, and 6 patients who had switched from Allo-SED to Auto-SED, reported that both types of SED had comparable tolerability and efficacy, and also proved to be equally safe [7]. In 2014, Harritshøj et al. reported on the evaluation of the Allo-SED service in Denmark [8] in 34 patients, noting that no side effects had been observed and a significant objective and subjective improvements in 16 out of 20 patients with keratoconjunctivitis sicca. No improvement, however, was noted in patients with PEDs during the treatment period [8].
While there are multiple reports of SED usage, these are generally small-scale studies. This is unsurprising, given that SED are generally prescribed as a treatment of last resort for patients who are refractory to conventional treatment and consequently has led to a situation where the clinical evidence underpinning SED treatment is limited. A recent Cochrane Database review focussing on Auto-SED [9] concluded that ‘Well-planned, large, high-quality RCTs are warranted to examine participants with dry eye of different severities by using standardized questionnaires to measure participant-reported outcomes, as well as objective clinical tests and objective biomarkers to assess the benefit of [Auto-SED] therapy for dry eye’. A similar conclusion was drawn by The Royal College of Ophthalmologists guidelines on SED for the treatment of severe OSD [4].
NHSBT established its Auto-SED service in 2003 following a small-scale controlled, crossover trial [10]. It became clear, however, that a significant proportion of patients who were referred for Auto-SED were of poor health, due to severe underlying medical conditions and were unable to donate their own blood. This resulted in patients being clinically disadvantaged and drove the implementation of its Allo-SED service in 2014. The NHSBT SED service is available to patients throughout the UK.
In this study, we report an analysis of patient-reported outcome measures (PROMs) in patients enrolled to the NHSBT SED programme for the management of severe OSD, to determine the patient benefit of SED usage, and whether there was a difference in patient characteristics and performance between patients treated with Auto-SED compared to those treated with Allo-SED.
Materials and methods
NHSBT serum eye drops service
Clinical requests are submitted to NHSBT SED programme predominantly by consultant ophthalmologists. They may refer patients for treatment with either Auto-SED or Allo-SED. Following initial assessment of the medical information by the NHSBT clinical team, patients referred for Auto-SED may not be accepted onto this Auto-SED programme if it is deemed unsafe for them to donate blood and subject to the approval of the referring clinician, these patients may be accepted into the Allo-SED programme.
Blood is collected from patients (Auto-SED), or for the allogeneic programme (Allo-SED), from healthy volunteer blood donors and processed into SED as previously described [10]. Briefly, the donated blood (approximately 450 ml) is allowed to clot, and the serum separated from the red cells by manual expression, followed by centrifugation to remove any residual red cells. The serum is then diluted with physiological saline (50% v/v), and aliquoted into 3 ml vials. The same collection and preparation protocol is used for both Auto-SED and Allo-SED. The prepared SED batch is packed in dry ice and delivered directly to the patient’s residence by the same day courier. Upon receipt, the patient transfers the SED to their domestic freezer. Prior to use, each frozen SED vial is allowed to thaw at room temperature. The patient then applies the SED as instructed by the referring clinician. This involves multiple dosing in both eyes according to the instructions of the prescribing ophthalmologist, using one vial per day. The default dosing instruction is every 4 h, although patients commencing treatment with SED usually have severe OSD and may need to use apply the SED more frequently initially. All patients are instructed that any residual SED remaining in the vial after 24 h must be discarded.
Data collection
PROMs were collected using the Ocular Surface Disease Index© (OSDI) (Allergan plc, Irvine, CA, USA) validated questionnaire, which can be either self or examiner administered without impacting on the outcome score (Appendix 1) [11]. The OSDI questionnaire poses a series of 12 items in three domains (symptoms, functional limitations and environmental factors) that the patient answers to grade the frequency of their symptoms and effect on vision-related function. After completion of the questionnaire, the answers are collated and scored according to the OSDI algorithm, which takes account of any questions not answered due to irrelevance, with a score of 100 representing the maximum severity of symptoms and a score of 0 representing no symptoms.
In addition to PROMs, patient demographic data including gender, age and underlying clinical diagnosis were collected and referring clinicians were asked whether or not SED treatment had been discontinued and, whether any adverse events and reactions had occurred.
PROM data were collected by NHSBT staff, predominantly by telephone; however, where it was not possible to contact patients by phone, or if they preferred to complete the questionnaire in their own time, forms were sent out by post with clear instructions regarding how to complete the questionnaire together with a stamped addressed envelope. Baseline and follow-up PROMs were collected retrospectively. This report analyses the dataset collected from patients who commenced treatment with either Auto-SED or Allo-SED between 1 January 2017 and 30 September 2018.
Appendix 2 shows the organisations who submitted data.
Data analysis
All data collected were uploaded into Microsoft Excel (Version 16.011929.20708, Microsoft Corporation, Washington, DC, USA). Data relating to patient demographics, treatment discontinuation, adverse events and reactions and underlying diagnoses were extracted and presented in a tabular and histogram formats.
Analysis of PROM (OSDI) scores was restricted to patients for whom two measures of OSDI were available, at baseline and at follow-up, approximately 12 months later. This meant that measures were clustered within individuals. To account for this non-independence of measures within individuals, a linear mixed model with two levels, measurement occasion (level 1) within individuals (level 2), was used for OSDI score, with a random intercept term at the individual level. The model included fixed effects of SED type (Auto-SED or Allo-SED) and measurement time (baseline or follow-up), as well as an interaction between the two, to allow for the change in OSDI score over time to differ between the Auto-SED and Allo-SED groups. To adjust for any differences in OSDI score or change in OSDI score due to the length of time between baseline and follow-up, the analysis also included the time between baseline and follow-up in months and an interaction between this and the categorical measurement time variable as fixed effects.
Given that the OSDI score is an ordinal variable over a sufficiently large scale (0–100), it was determined that it was acceptable to treat it as a numerical (non-categorical) variable. Maximum likelihood estimation was used to fit the linear mixed model as our main interest was in estimation of the fixed effects. All statistical analyses were performed using SAS Enterprise Guide v7.1 software (SAS Institute Inc., Cary, NC, USA).
Results
Over the designated time period, 318 patients commenced treatment with SED, of whom 279 received either Auto-SED or Allo-SED and were included in the further analyses. The remaining 38 patients were excluded as they had received a combination (not simultaneously) of Auto-SED and Allo-SED during the designated time period. Of these, 24 patients were initially assigned to receive Auto-SED but were subsequently transferred to Allo-SED, principally due to health-related issues that prevented them from donating their own blood. A further 14 patients were receiving Auto-SED, but had also received at least one batch of Allo-SED, due to loss of an autologous donation due technical issues during processing, or had to start on Allo-SED due to an urgent medical need supply of SED, then changed to Auto-SED.
To minimise confounding factors, subsequent analysis was restricted to the 279 patients who had received one type of SED, either Auto-SED or Allo-SED alone, during the study period. There were 71 patients (45 females) who received Auto-SED and 208 patients (125 females) who received Allo-SED, with a mean age for Auto-SED patients of 53 (SD ± 15) versus 56 (SD ± 20) for Allo-SED (p = 0.12)
Discontinuation of treatment, adverse reactions and adverse events
This information was reported back by the referring clinicians for 155 of the 279 patients who had received either Auto-SED or Allo-SED (40 Auto-SED and 115 Allo-SED).
Eleven (7%) patients discontinued SED treatment (three Auto-SED and eight Allo-SED) for a variety of reasons as summarised in Table 1.
Adverse events/reactions were reported in three patients (one Auto-SED and two Allo-SED) whilst on treatment. In these patients, the stated adverse event/reaction (i.e., corneal graft failure, severe chest infection, aseptic ulcer) were considered to be unlikely to be caused by the SED treatment.
Indications for SED treatment
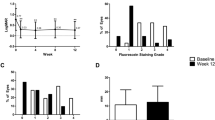
The diagnostic groups for patients commencing SED treatment are detailed in Fig. 1. There was no difference in the clinical indication for Auto-SED versus Allo-SED, although Sjögren’s patients were more likely to receive Auto-SED and those with other immune-related diseases (OcMMP, SJS–TEN, GVHD) were more likely to receive Allo-SED due to systemic comorbidities.
(1) Ocular mucous membrane pemphigoid, Stevens–Johnson syndrome, toxic epidermal necrolysis, graft-versus-host disease, other immune-related dry eye. (2) Meibomian gland disease, other non-immune dry eye. (3) Diabetic cornea, herpetic aetiology, other neurotropic keratopathy. (4) Ocular surface toxicity, thermal, chemical, mechanical, surgical, radiation, other types of injury/trauma. (5) ITU/HDU patient, thyroid-associated ophthalmopathy, non-thyroid proptosis, other types of exposure keratopathy. (6) Ocular surface reconstruction, cornea transplant, other supportive indications. (7) Aniridia, ectodermal dysplasia, epidermolysis bullosa, other types of inherited ocular surface diseases. (8) Main indication not specified by referring clinician.
Analysis of PROM data
The linear mixed model assumes normality of residuals, and therefore residual plots were checked for deviations from normality. The model was fitted using the total OSDI score on its original scale as the outcome variable, as well as using the square and square root of OSDI score as the outcome. Residuals appeared reasonably normally distributed using the untransformed OSDI score and the transformed OSDI scores appeared to fit a normal distribution less well, although findings were not meaningfully changed using the transformed scores. Results are therefore reported using the untransformed score.
The data are summarised in Fig. 2 as a box and whisker plot. The boxes range from the first quartile to the third quartile of the data, with the median values represented by the horizontal line through the middle of the box, and diamond symbols representing the mean values. The whiskers range from the lowest to the highest data points within 1.5 times the interquartile range below and above the box edges. One data point (in the Auto-SED follow-up group) is an outlier, i.e., it is further from the box than 1.5 times the interquartile range. This is represented by the small circle on the figure.
Table 2 shows descriptive statistics for OSDI scores at baseline and follow-up, the difference in these and the time between baseline and follow-up for participants in the Auto-SED and Allo-SED groups. There were 49 patients (69%) with complete information on baseline and follow-up OSDI score in the Auto-SED group and 112 (54%) in the Allo-SED group. Both groups showed a reduction, on average, in OSDI score between baseline and follow-up. For Auto-SED the mean reduction in OSDI score was 34.43 (SD 23.68) and for Allo-SED 29.85 (SD 22.04, two-sample t test p value for difference between the two groups = 0.24). The mean (SD) duration between baseline and follow-up was 12.6 (6.2) months in the Auto-SED group and 14.1 (6.1) months in the Allo-SED group (Mann–Whitney U test p value for difference between the two groups = 0.18).
A comparison of the change in mean OSDI score between follow-up and baseline in each of the SE groups using the least squares means from the linear mixed model is given in Table 3. This shows strong evidence of an average reduction in OSDI score in both Auto-SED and Allo-SED groups between baseline and follow-up (mean difference in Auto-SED group −34.58 [−40.90, 28.26], mean difference in Allo-SED group −30.34 [−34.67, −26.00] units; p = 0.27 for difference in change between groups).
Table 4 shows the estimates of fixed effects from the linear mixed model for OSDI score. This shows that there was no evidence of a difference in OSDI score at baseline between Allo-SED and Auto-SED patients (mean difference 5.23, 95% confidence interval (CI) [−2.20, 12.65] units). It also shows that in Auto-SED patients OSDI score reduced between baseline and follow-up (mean difference −34.58, 95% CI [−40.90, −28.26] units), but there was no evidence of a difference in this change in OSDI score over time between Allo-SED and Auto-SED patients (mean difference in change in OSDI score 4.24, 95% CI [−3.36, 11.85] units, evident by the interaction term between measurement time and SED group in the table). There was no evidence that the time between baseline and follow-up was associated with OSDI score at baseline or the change in OSDI score between baseline and follow-up.
Discussion
This is the largest case series reported to date evaluating the impact of SED on PROMs and shows equal performance of Allo-SED and Auto-SED.
Both Auto-SED and Allo-SED were well tolerated by the large majority of patients reported in this study. Over the follow-up period, 7% [11] patients who joined the SE programme (and for whom data were available) had treatment withdrawn and only 3% withdrew due to inability to tolerate SED (n = 3) or due to receiving no benefit from them (n = 2). Adverse events or reactions were reported in a rather low proportion (2%) of patients; however, following review none of these were considered likely to be related to SED treatment. These findings are consistent with the clinical trial underpinning our service [10] and the majority of other clinical studies that have investigated SED [4].
The data collected in this study were collated from 161 individuals and demonstrates a highly significant improvement in PROM as measured by the OSDI for patients treated with either Auto-SED or Allo-SED. The dataset shows an asymmetrical distribution of patients, with approximately twice as many receiving Allo-SED as Auto-SED, representing a real-world referral pattern for NHSBT SED service over the time period studied, with 64% of new referrals being for Allo-SED.
The data published in 2004 by Noble et al. [10] examined the benefits of Auto-SED in 16 patients demonstrating a significant improvement in PROMs using a Rasch weighted ‘faces’ scale. In this study we collected performance data using the OSDI© questionnaire [11], as it is a widely used quality of life measures questionnaire and has been shown to be a valid and reliable tool for quantifying the impact of dry eye disease [12]. It possesses the required psychometric properties to be used as an end point in clinical trials [12] and can be administered by either the patient or the examiner without invalidating the results. Celebi et al. [13] used the OSDI scale to measure improvements in 20 patients with severe dry eye syndrome treated with Auto-SED, reporting a mean decrease of 55% at 1-month follow-up. Similarly, Urzua et al. [14] reported data from 12 patients, noting a statistically higher decrease in OSDI score of 50% compared to those on standard treatment (22% reduction). These findings are similar to the 58% decrease in OSDI score that we observed in our Auto-SED patient group. We are not aware of any equivalent studies of the efficacy of Allo-SED at reducing OSDI score for comparison. In our study, we found a 46.5% reduction in OSDI score for patients treated with Allo-SED. The difference in decrease in OSDI score was not statistically significant between patients receiving Auto-SED and Allo-SED.
Our study analyses PROMs using the OSDI instrument in patients enrolled to the NHSBT SED programme for the management of severe OSD. It provides patient-benefit performance data on the use of SED provided by NHSBT in the form of an observational service cohort analysis rather than a formal clinical trial. Data interpretation is therefore limited by the absence of a control arm (standard care treatment) and randomisation, as patients were referred for either Auto-SED or Allo-SED treatment by the referring clinician. In addition, there was no masking to either Auto-SED or Allo-SED and this could introduce bias. Data collection, however, was undertaken independently of data analysis. There were no specific inclusion or exclusion criteria for patient involvement in this study, other than suitability for the SED programme, as determined by the referring clinicians. For the purposes of data collection and this report, existing patients already on the SED programme or those who switched between Auto-SED and Allo-SED during the course of the study were excluded.
There was variation in the way in which OSDI scores were collected; the majority were collected over the phone by trained members of our own team; however, some were done by patients themselves as written responses and returned by post. There were two reasons for this; in some cases, it was not possible to contact the patients by phone, and also some patients preferred to complete the questionnaires in their own time. It must be acknowledged that this variation in the method of data collection and the personnel collecting the data could introduce bias, although efforts were made to minimise this; for example, for consistency, all staff involved in data collection were trained to use a standard script; and those of an older age group are more likely to respond positively through an examiner administered questionnaire than self-administered questionnaire due to a number of reasons including presbyopia, blurred vision from the OSD, compounding their ability to self-administer the questionnaire. It should also be noted that retrospective collection of baseline PROMs is not ideal and may have introduced bias due to patient recall of symptoms prior to initiating SED treatment. It must also be considered that during the course of the study, all patients were using some form of adjunctive treatments (e.g., ointment at night, carbomer gel, among others) in addition to SEDs, which may also have impacted on their OSDI scores. SED, however, is, in most cases, the main treatment. This is an unavoidable consequence stemming from the observational nature of the study. As per Royal College of Ophthalmologists Guidelines [4], patients were only enrolled in the SED programme when most/all classes of adjunct treatment had been explored. It is expected that all patients were still using some/few adjunctive therapy, as it is extremely rare to rely on SED only. However, there is however no reason to suggest that either treatment group was more or less likely to use adjunctive treatments
Finally, it was not possible to collect baseline and follow-up data from all patients who commenced treatment with SED in the designated time period and a full dataset was collected from 161 of 279 potential participants (58%). There were multiple reasons for non-collection, principally an inability to contact or obtain a response from patients. While acknowledging these limitations, the value of data collection in a real-time evaluation setting with standardised questionnaire provides support for the patient benefit from the SED treatment.
A further source of variation is the inter person/donor variation in the biological composition of the serum, leading to the possibility that some batches may be less effective than others. It has been demonstrated that there is considerable variation in the levels of growth factors and cytokines in serum prepared from different patients and donors, and that these differences can be associated with different clinical outcomes [15]. A potential option to smooth out these variations that may be applied to Allo-SED is pooling of donations from multiple donors prior to preparation of SED. Further studies are required to explore the potential risks and benefits of this approach.
In conclusion, despite these limitations, we have demonstrated that treatment with both Auto-SED and Allo-SED leads to a significant reduction in the severity of symptoms experienced by patients, confirming the findings previously reported in much smaller groups of patients and finding a similar magnitude of improvements. In terms of PROMs, no significant difference was found between the degree of improvement following treatment with both Auto-SED and Allo-SED. Both types of SED were well tolerated by patients, and no SED-related adverse events or reactions were recorded throughout the study. A large-scale, randomised, controlled trial to compare treatment with Auto-SED, Allo-SED with a control arm will provide more robust evidence for the benefits of SED treatment, as recommended by the Cochrane Review [9] and The Royal College of Ophthalmologists [4].
Summary
What was known before
-
Serum eye drops can be highly effective in mitigating the symptoms of severe ocular surface disease. Serum can be taken from the patient themselves, or volunteer donors.
-
There is a lack of good-quality clinical data demonstrating that serum eye drops provide significant improvements in patient-reported outcomes.
-
There are no comparative studies of the efficacy of autologous and allogeneic serum eye drops.
What this study adds
-
Both autologous and allogeneic serum eye drops significantly improve patient-reported outcome measures in patients affected by severe ocular surface disease.
-
Both types of serum eye drop are equally effective.
References
Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EC, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510.
Buchholz P, Steeds CS, Stern LS, Wiederkehr DP, Doyle JJ, Katz LM, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf. 2006;4:155–61.
Fox RI, Chan R, Michelson JB, Belmont JB, Michelson PE. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984;27:459–61.
Rauz S, Koay SY, Foot B, Kaye SB, Figueiredo F, Burdon MA, et al. The Royal College of Ophthalmologists guidelines on serum eye drops for the treatment of severe ocular surface disease: executive summary. Eye. 2018;32:44–8.
Chiang CC, Lin JM, Chen WL, Tsai YY. Allogeneic serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Cornea. 2007;26:861–3.
Chiang CC, Chen WL, Lin JM, Tsai YY. Allogeneic serum eye drops for the treatment of persistent corneal epithelial defect. Eye (Lond). 2009;23:290–3.
Hung Y, Elder MJ, Rawstron JA, Badami KG. A retrospective crossover study of autologous and allogeneic serum eye drops for the management of ocular surface disease. Transfus Med. 2019;29:69–71.
Harritshøj LH, Nielsen C, Ullum H, Hansen MB, Julian HO. Ready-made allogeneic ABO-specific serum eye drops: production from regular male blood donors, clinical routine, safety and efficacy. Acta Ophthalmol. 2014;92:783–6.
Pan Q, Angelina A, Marrone M, Stark WJ, Akpek EK. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2017;2:CD009327.
Noble BA, Loh RS, MacLennan S, Pesudovs K, Reynolds A, Bridges LR, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88:647–52.
Walt JG, Rowe MM, Stern KL. Evaluating the functional impact of dry eye: the Ocular Surface Disease Index. Drug Inf J. 1997;31:1436.
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21.
Celebi AR, Ulusoy C, Mirza GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthalmol. 2014;252:619–26.
Urzua CA, Vasquez DH, Huidobro A, Hernandez H, Alfaro J. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Curr Eye Res. 2012;37:684–8.
Campos E, Versura P, Buzzi M, Fontana L, Giannaccare G, Pellegrini M, et al. Blood derived treatment from two allogeneic sources for severe dry eye associated to keratopathy: a multicentre randomised cross over clinical trial. Br J Ophthalmol. 2020;104:1142–7.
Acknowledgements
The authors would like to thank Dr. J. B. Mueller, Dr. L. Gaum, J. Leyland and J. Dobie for their help with data collection for this study, D. Stinson for secretarial support and Dr. E. Allen, Principal Statistician, Statistics and Clinical Studies NHS Blood and Transplant for her help with the statistical analyses.
Author information
Authors and Affiliations
Contributions
RJL, AC, SR, SK and FCF were responsible for designing the study protocol. RJL was the primary author of the manuscript, with input provided by AC, SR, SK and FCF. CMcW developed the statistical analysis protocol and carried out all analyses.
Corresponding author
Ethics declarations
Conflict of interest
RJL, AC and CMcW are employees of NHS Blood and Transplant, accredited suppliers of the serum eye drops service in the UK.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lomas, R.J., Chandrasekar, A., Macdonald-Wallis, C. et al. Patient-reported outcome measures for a large cohort of serum eye drops recipients in the UK. Eye 35, 3425–3432 (2021). https://doi.org/10.1038/s41433-021-01560-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01560-8
This article is cited by
-
Allogeneic Serum Eye Drops: A Randomized Clinical Trial to Evaluate the Clinical Effectiveness of Two Drop Sizes
Ophthalmology and Therapy (2023)