Abstract
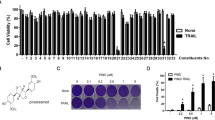
Allantopyrone A is an α-pyrone metabolite that was originally isolated from the endophytic fungus Allantophomopsis lycopodina KS-97. We previously demonstrated that allantopyrone A exhibits anti-cancer, anti-inflammatory, and neuroprotective activities. In the present study, we showed that allantopyrone A up-regulated the protein expression of hypoxia-inducible factor (HIF)-1α in human fibrosarcoma HT-1080 cells. It also up-regulated the mRNA expression of BNIP3 and ENO1, but not other HIF target genes or HIF1A. Allantopyrone A did not inhibit the prolyl hydroxylation of HIF-1α, but enhanced the ubiquitination of cellular proteins. Consistent with this result, chymotrypsin-like and trypsin-like proteasome activities were reduced, but not completely inactivated by allantopyrone A. Allantopyrone A decreased the amount of proteasome catalytic subunits. Therefore, the present results showed that allantopyrone A interfered with the degradation of HIF-1α protein by reducing proteasome activity in human fibrosarcoma HT-1080 cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408.
Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402.
Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75.
Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309.
Fong GH, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008;15:635–41.
Wong BW, Kuchnio A, Bruning U, Carmeliet P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem Sci. 2013;38:3–11.
Ivan M, Kaelin WG Jr. The EGLN-HIF O2-sensing system: multiple inputs and feedbacks. Mol Cell. 2017;66:772–8.
Shen C, Kaelin WG Jr. The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2013;23:18–25.
Gossage L, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15:55–64.
Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–28.
LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat. Cell Biol. 2016;18:356–65.
Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)α: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12.
Baldewijns MM, van Vlodro IJH, Vermeulen PB, Soetekouw PMMB, van Engeland M, et al. VHL and HIF signalling in renal cell carcinogenesis. J Pathol. 2010;221:125–38.
Shiono Y, Yokoi M, Koseki T, Murayama T, Aburai N, et al. Allantopyrone A, a new α-pyrone metabolite with potent cytotoxicity from an endophytic fungus, Allantophomopsis lycopondina KS-97. J Antibiot. 2010;63:251–3.
Schüffler A, Liermann JC, Opatz T, Anke T. Elucidation of the biosynthesis and degradation of allantofuranone by iso-topic labelling and fermentation of modified precursors. ChemBioChem. 2011;12:148–54.
Elsbaey M, Tanaka C, Miyamoto T. Allantopyrone E, a rare α-pyrone metabolite from the mangrove derived fungus Aspergillus versicolor. Nat Prod Res. 2022;36:760–4.
Uesugi S, Muroi M, Kondoh Y, Shiono Y, Osada H, et al. Allantopyrone A activates Keap1-Nrf2 pathway and protects PC12 cells from oxidative stress-induced cell death. J Antibiot. 2017;70:429–34.
Yokoigawa J, Morimoto K, Shiono Y, Uesugi S, Kimura K, et al. Allantopyrone A, an α-pyrone metabolite from an endophytic fungus, inhibits the tumor necrosis factor α-induced nuclear factor κB signaling pathway. J Antibiot. 2015;68:71–5.
Quach HT, Tanigaki R, Yokoigawa J, Yamada Y, Miwa M, et al. Allantopyrone A interferes with multiple components of the TNF receptor 1 complex and blocks RIP1 modifications in the TNF-α-induced signaling pathway. J Antibiot. 2017;70:929–36.
Kondo T, Takeda K, Muko R, Ito A, Chang YC, et al. 4-O-Methylascochlorin inhibits the prolyl hydroxylation of hypoxia-inducible factor-1α, which is attenuated by ascorbate. J Antibiot. 2019;72:271–81.
Zhang Y, Lian F, Zhu Y, Xia M, Wang Q, et al. Cyanidin-3-O-β-glucoside inhibits LPS-induced expression of inflammatory mediators through decreasing IκBα phosphorylation in THP-1 cells. Inflamm Res. 2010;59:723–30.
Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, et al. Recruitment of HIF-1α and HIF-2α to common target genes is differently regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell. 2006;10:413–23.
Spirina LV, Yurmazov ZA, Gorbunov AK, Usynin EA, Lushnikova NA, et al. Molecular protein and expression profile in the primary tuors of clear cell renal carcinoma and metastases. Cells. 2020;9:1680.
Konieczna A, Szczepańska A, Sawiuk K, Węgrzyn G, Łyżeń R. Effects of partial silencing of genes coding for enzymes involved in glycolysis and tricarboxylic acid cycle on the enterance of human fibroblasts to the S phase. BMC Cell Biol. 2015;16:16.
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypotoxic cells. Cell. 2007;129:111–21.
Gillespie DL, Whang K, Ragel BT, Flynn JR, Kelly DA, et al. Silencing of hypoxia inducible factor-1α by RNA interference attenuates human glioma cell growth in vivo. Clin Cancer Res. 2007;13:2441–8.
Sun X, Huang Q, Peng F, Wang J, Zhao W, et al. Expression and clinical significance of HKII and HIF-1α in grade groups of prostate cancer. Front. Genet. 2021;12:680928.
Park HS, Kim JH, Sun BK, Song SU, Suh W, et al. Hypoxia induces glucose uptake and metabolism of adipose-derived stem cells. Mol Med Rep. 2016;14:4706–14.
Karagiota A, Kanoura A, Paraskeva E, Simos G, Chachami G Pyruvate dehydrogenase phosphatase 1 (PDP1) stimulates HIF activity by supporting histone acetylation under hypoxia. FEBS J. 2022, in press, https://doi.org/10.1111/febs.16694.
Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol. Cell. 2007;28:941–53.
Futamura Y, Muroi M, Aono H, Kawatani M, Hasashida M, et al. Bioenergetic and proteomic profiling to screen small molecule inhibitors that target metabolisms. Biochim Biophys Acta Proteins Proteom. 2019;1867:28–37.
Moon JY, Miyazaki T, Muroi M, Watanabe N, Shin R. Isolation of novel chemical components and their plant garget proteins under selenium stress. Methods Enzymol. 2023;680:421–38.
Chinnadurai G, Vijayalingam S, Gibson SB. BNIP3 subfamily BH3-only proteins: mitochondrial stress sensors in normal and pathological functions. Oncogene. 2009;27:S114–27.
Pagès G, Pouysségur J. Transcriptional regulation of the vascular endothelial growth factor gene–a concert of activating factors. Cardiovasc Res. 2005;65:564–73.
Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–13.
Thibaudeau TA, Smith DM. A practical review of proteasome pharmacology. Pharmacol Rev. 2019;71:170–97.
Besche HC, Haas W, Gygi SP, Goldberg AL. Isolation of mammalian 26S proteasomes and p97/VCP complexes using the ubiquitin-like domain from HHR23B reveals novel proteasome-associated proteins. Biochemistry. 2009;48:2538–49.
Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–90.
Harer SL, Bhatia MS, Bhatia NM. Proteasome inhibitors mechanism; source for design of newer therapeutic agents. J Antibiot. 2012;65:279–88.
Kataoka T. Chemical biology of inflammatory cytokine signaling. J Antibiot. 2009;62:655–67.
Acknowledgements
The authors would like to thank Dr. Takehiro Suzuki (RIKEN CSRS) and Dr. Naoshi Dohmae (RIKEN CSRS) for protein identification. This work was partly supported by JSPS KAKENHI Grant number 19H02885 (to T.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okuda, C., Ueda, Y., Muroi, M. et al. Allantopyrone A interferes with the degradation of hypoxia-inducible factor 1α protein by reducing proteasome activity in human fibrosarcoma HT-1080 cells. J Antibiot 76, 324–334 (2023). https://doi.org/10.1038/s41429-023-00610-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00610-5