Abstract
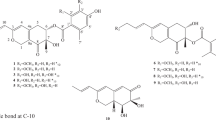
Two new naphthyl-products calinaphthyltriol A (1) and calinaphthalenone A (2) were isolated from Aspergillus californicus IBT 16748 together with one known compound ophiobolin X (3). Their structures were elucidated by extensive spectroscopic analyses. The absolute configuration of 2 was solved by comparing its optical rotation with data for the known compounds 4, 5, and 6 as well as theoretical calculations. The antibacterial and cytotoxic activities of 1 and 3 were evaluated. Both compounds did not show antibacterial activity (MIC > 96 µg·ml−1) against a few selected clinically relevant Gram positive and Gram negative bacterial strains. However, they showed moderate cytotoxicity against HL-60 cell line with IC50 values of 18 and 24 µg·ml−1, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Frisvad JC, Larsen TO. Chemodiversity in the genus Aspergillus. Appl Microbiol Biotechnol. 2015;99:7859–77.
Alburae NA, Mohammed AE, Alorfi HS, Jamanturki A, Asfour HZ, Alarif WM, et al. Nidulantes of Aspergillus (Formerly Emericella): A treasure trove of chemical diversity and biological activities. Metabolites. 2020;10:1–28.
Sanchez JF, Somoza AD, Keller NP, Wang CCC. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat Prod Rep. 2012;29:351–71.
Samson RA, Varga J, Meijer M, Frisvad JC. New taxa in Aspergillus section Usti. Stud Mycol. 2011;69:81–97.
Chen AJ, Frisvad JC, Sun BD, Varga J, Kocsubé S, Dijksterhuis J, et al. Aspergillus section Nidulantes (formerly Emericella): Polyphasic taxonomy, chemistry and biology. Stud Mycol. 2012;84:1–118.
Zhu T, Lu Z, Fan J, Wang L, Zhu G, Wang Y, et al. Ophiobolins from the mangrove fungus Aspergillus ustus. J Nat Prod. 2018;81:2–9.
Tsukamoto H, Sato M, Kondo Y. Palladium(0)-catalyzed direct cross-coupling reaction of allyl alcohols with aryl- and vinyl-boronic acids. Chem Commun. 2004;10:1200–1.
Zhou YH, Zhang M, Zhu RX, Zhang JZ, Xie F, Bin LiX, et al. Heptaketides from an endolichenic fungus Biatriospora sp. and their antifungal activity. J Nat Prod. 2016;79:2149–57.
Quideau S, Lyvinec G, Marguerit M, Bathany K, Ozanne-Beaudenon A, Buffeteau T, et al. Asymmetrie hydroxylative phenol dearomatization through in situ generation of iodanes from chiral iodoarenes and m-CPBA. Angew Chem - Int Ed. 2009;48:4605–9.
Cacho RA, Jiang W, Chooi YH, Walsh CT, Tang Y. Identification and characterization of the echinocandin b biosynthetic gene cluster from Emericella rugulosa NRRL 11440. J Am Chem Soc. 2012;134:16781–90.
Wavefunction Inc. Spartan. Irvine, CA: Wavefunction Inc. 2020. https://www.wavefun.com/.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 16. Wallingford CT: Gaussian, Inc. 2016.
Mennucci B, Tomasi J, Cammi R, Cheeseman JR, Frisch MJ, Devlin FJ, et al. Polarizable Continuum Model (PCM) calculations of solvent effects on optical rotations of chiral molecules. J Phys Chem A. 2002;106:6102–13.
Rossi D, Nasti R, Collina S, Mazzeo G, Ghidinelli S, Longhi G, et al. The role of chirality in a set of key intermediates of pharmaceutical interest, 3-aryl-substituted-γ-butyrolactones, evidenced by chiral HPLC separation and by chiroptical spectroscopies. J Pharm Biomed Anal. 2017;10:41–51.
Mazzeo G, Cimmino A, Masi M, Longhi G, Maddau L, Memo M, et al. Importance and difficulties in the use of chiroptical methods to assign the absolute configuration of natural products: the case of phytotoxic pyrones and furanones produced by diplodia corticola. J Nat Prod. 2017;80:2406–15.
Acknowledgements
The authors thank Dr. Kasper Enemark-Rasmussen and Associate Professor René Wugt Larsen from Department of Chemistry at the Technical University of Denmark for acquiring NMR and IR data, respectively. YG is financially supported by the China Scholarship Council and Department of Biotechnology and Biomedicine at the Technical University of Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Guo, Y., Ghidinelli, S., de la Cruz, M. et al. New naphthyl derivatives from Aspergillus californicus. J Antibiot 74, 111–114 (2021). https://doi.org/10.1038/s41429-020-00372-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-00372-4