Abstract
Bone tissue engineering is pivotal in facilitating bone reconstruction by promoting persistent angiogenesis and osteogenesis. Initially, the hot gel composite hydrogel scaffold technique was employed. However, to address various limitations, numerous gel structures have since been developed, including osteogenic gellan gels, semi-interpenetrating network hydrogels, photoinduced crosslinking methacrylate gels, and supramolecular hydrogels. This review examines the mechanisms, formation principles, and medical benefits of these gel structures. In addition, novel bioengineering techniques to regulate human bone growth are expected to emerge in the future. This work is expected to significantly expedite the advancement of hydrogel membranes in the field of bone repair.
Similar content being viewed by others
Introduction
A hydrogel is a three-dimensional (3D) network structure composed of hydrophilic polymer chains with a high content ranging from 90% to 99%, which facilitates efficient oxygen and substance exchange1. In recent decades, a variety of natural hydrogels (e.g., alginate (Alg)/gelatin) and synthetic hydrogels (e.g., gelatin-PEG and PAAm/Dex-U) have undergone unprecedented development in biomedical fields due to their high porosity, satisfactory nutritional permeability, high biocompatibility, low immunogenicity, and tunable physical and chemical properties2,3,4. Compared to other organic molecules or polymers, the multimolecular structure of hydrogels can provide a suitable matrix for cell transplantation and differentiation, endogenous regeneration, biorepair, wound healing and continuous drug delivery5,6,7,8. Moreover, the 3D network system of hydrogels can simulate the microstructure of the extracellular matrix (ECM) and provide a suitable environment for cell survival9,10,11,12.
In contemporary society, diseased or damaged bone tissue is a common clinical problem in orthopedic medicine, and bone has become the second most frequently transplanted tissue after blood13,14,15. Although both nonsurgical (e.g., casting, electrical stimulation, and ultrasound therapy) and surgical approaches (e.g., internal fixation, external fixation, bone grafting and bone regeneration) have been applied for bone repair, these existing treatments may not be suitable for all types of bone diseases16. For example, autologous or allogeneic bone transplantation is commonly used for treating bone defects; however, this therapy can cause damage at the donor site, and acquiring suitable donors can be challenging17,18. To address the aforementioned problems, bone tissue engineering utilizing hydrogels or hydrogel membranes as scaffolds has emerged as a promising solution. To the best of our knowledge, hydrogels composed of crosslinked polymer chains represent a unique class of scaffold materials characterized by a 3D hydrophilic network structure that can maintain its stability even after absorbing several times its own volume of water19,20,21. Therefore, hydrogels can mimic the natural tissue environment and provide structural support to defect sites, allowing bone defects to be repaired by intrinsic healing mechanisms22.
Despite years of exploration, there are still many unsolved problems regarding the use of hydrogels or hydrogel membranes for bone repair. The greatest challenges are as follows: (1) During the fabrication of synthetic hydrogels, potentially toxic crosslinkers may be utilized. (2) Most synthetic procedures are intricate and laborious. (3) Although natural hydrogels are considered ideal for bone repair, their poor mechanical properties often hinder their application in this field. Therefore, effective strategies for constructing suitable gels that promote cell adhesion and growth are urgently needed.
In this review, we first present a fundamental overview of bone repair, encompassing nonsurgical modalities. (casting, electrical stimulation, ultrasound therapy) and surgical approaches (internal fixation, external fixation, bone grafting and bone regeneration). The present study provides a comprehensive overview of the recent advances and challenges in the application of hydrogels for bone repair. Various hydrogel structures, including osteogenic gellan gel, semi-interpenetrating network hydrogels (semi-IPNs), interpenetrating network hydrogels (IPNs), and photoinduced crosslinking methacrylate gelatin (MAGel), are highlighted. The functional, mechanistic, and medical advantages of these hydrogel structures are thoroughly examined (Fig. 1). The findings of this study suggest that further research will lead to the development of more advanced hydrogel materials and bioengineering techniques for bone repair.
Profiles of bone repair
Before discussing the role of hydrogels in bone repair, we first describe and categorize several commonly used techniques for bone repair. Basically, techniques for bone repair can be broadly classified into nonsurgical and surgical approaches. The nonsurgical approaches include casting, electrical stimulation, and ultrasound therapy, and the surgical approaches include internal fixation, external fixation, bone grafting and bone regeneration (Fig. 2). Notably, existing nonsurgical or surgical approaches may not be suitable for all types of bone fractures. The severity of the fracture, the age and overall health of the patient, and other factors determine the most appropriate treatment plan. Detailed descriptions are provided below.
Nonsurgical approaches
Casting
Casting involves using a cast or brace to immobilize the broken bone and allow it to heal naturally. A cast is commonly made of rigid material, such as plaster or fiberglass, which is wrapped around the affected limb to keep the bone in place. The cast is usually worn for several weeks to months, depending on the severity of the fracture and the individual’s healing progress23,24,25,26,27,28. Generally, casting is a nonsurgical approach that is commonly used for bone fractures29,30,31,32,33,34 and typically involves the following steps: initial evaluation, reduction, and immobilization35,36,37,38.
Electrical stimulation
Electrical stimulation is a noninvasive approach that promotes bone healing by stimulating the activity of bone cells. Electrical stimulation can be administered in several ways, including direct current stimulation (DCS), pulsed electromagnetic field (PEF) stimulation, or capacitive coupling (CC)39,40,41. DCS involves the application of a small electrical current to the skin or directly to the bone, which is thought to stimulate the activity of bone cells and promote the growth of new bone tissue42. PEF and CC involve the use of low-frequency electromagnetic waves around the affected area43,44,45,46,47,48.
Ultrasound therapy
Ultrasound therapy is a noninvasive approach in which high-frequency sound waves are used to stimulate bone healing. The sound waves penetrate deep into the tissue and stimulate the activity of bone cells, thereby promoting the growth of new bone tissue49,50,51,52. There are two types of ultrasound therapy: low-intensity pulsed ultrasound (LIPUS) and high-intensity focused ultrasound (HIFU). LIPUS uses low-intensity sound waves to promote bone healing, while HIFU uses high-intensity sound waves to destroy damaged tissue and stimulate new tissue growth53.
Summary
The nonsurgical approaches of casting, electrical stimulation, and ultrasound therapy all possess potential risks and limitations (Table 1). For instance, the cast may need to be removed or replaced if it becomes too tight or if the skin underneath becomes irritated or infected. Electrical stimulation and ultrasound therapy are often used in conjunction with other bone repair techniques, such as casting or bone grafting, to help accelerate the healing process54. However, these approaches may not be suitable for all types of bone fractures or medical conditions. A qualified health care professional should be consulted to determine the best course of action for a particular individual.
Surgical approaches
Internal fixation
Internal fixation is a surgical technique in which bone fractures are repaired by implanting metal screws, plates, or rods inside the body to hold the broken bone in place. These implants are typically made of titanium or stainless steel and are designed to remain in the body permanently or temporarily55,56,57. Internal fixation is usually recommended for complex fractures or for fractures that cannot be treated with nonsurgical approaches. The procedure involves making an incision at the site of the fracture and using specialized tools to position the implants accurately58,59.
External fixation
External fixation is a surgical technique in which bone fractures are repaired by implanting metal pins or screws into the bone and attaching them to an external metal frame that holds the bone in place. The pins or screws are inserted through the skin and into the bone on either side of the fracture, and the external frame is attached to the pins or screws60. External fixation is typically recommended for complex fractures that cannot be treated with nonsurgical approaches such as casting or internal fixation61.
Bone grafting
Bone grafting is a surgical technique in which bone fractures are repaired by transplanting bone tissue from one area of the body (called the donor site) to the area of the fracture (called the recipient site). Bone grafting is typically used when a fracture is severe, has failed to heal properly, or is located in an area with poor blood supply62,63,64. There are several types of bone grafts, including autografts (bone tissue from the patient’s own body), allografts (bone tissue from a donor), and synthetic grafts (artificial materials)65. During the procedure, a surgeon makes an incision at the site of the fracture and removes all damaged or dead tissue, then transplants the bone tissue from the donor site to the recipient site and secures it using pins, screws, or plates66,67,68.
Bone regeneration
Bone regeneration is the process by which new bone tissue is formed to replace damaged or missing bone tissue. This process is stimulated by growth factors and other biological agents, which can be applied directly to the site of a bone injury or defect69,70,71. Bone regeneration can occur naturally, but it can also be enhanced through the use of biological agents such as bone morphogenetic proteins (BMPs), growth factors, and stem cells. BMPs are naturally occurring proteins that stimulate the formation of new bone tissue. Growth factors are proteins that promote cell growth and differentiation and can help stimulate the growth of new bone tissue. Stem cells are undifferentiated cells that can differentiate into bone cells and thereby help to regenerate bone tissue72,73,74. In addition to biological agents, scaffolds or matrices can be used to facilitate bone regeneration. These 3D structures provide a template on which new bone tissue can grow. They can be made from natural materials (e.g., collagen) or synthetic materials (e.g., hydrogels)75,76,77,78,79. Polymeric hydrogels with a structure analogous to the extracellular matrix (ECM) have been recognized as promising platforms for loading various biological agents to promote bone regeneration.
Summary
Surgical approaches for bone repair involve physically manipulating the bones to realign them and/or using implants to hold them in place while they heal. Surgery may be necessary to treat severe bone fractures or fractures for which nonsurgical approaches are ineffective. In surgical treatment, the multimolecular system of hydrogels exhibits promise as an appropriate matrix for cell transplantation and differentiation, endogenous regeneration, biorepair, wound healing, and continuous drug delivery, which is attributed to the 3D microstructures that closely resemble the original ECM network system. It should be noted that surgical approaches to bone repair also carry some risks, such as infection, bleeding, inflammation, nerve damage and rejection reactions (Table 1). The recovery time after surgery can also depend on various factors, such as the extent of the injury, the overall health status of the patient, and the quality of the bone tissue at the injury site.
Functions and underlying mechanisms of hydrogels in bone repair
Hydrogels, water-swollen crosslinked polymer networks, have attracted increasing interest in the field of bone repair due to their biocompatibility, biodegradability, and tunable mechanical and physicochemical properties80. On the basis of previous reports, we divide the functions and mechanisms of hydrogels in bone repair into several categories, as hydrogels or hydrogel membranes can serve as scaffolds for cell delivery, drug delivery systems, tissue engineering materials, bone substitutes, and implant coatings.
Scaffolds for cell delivery
Hydrogels can be used as scaffolds for cell delivery in bone repair, providing a 3D environment that supports cell growth and proliferation. The hydrogel scaffold can be loaded with bone-forming cells, such as mesenchymal stem cells (MSCs), and implanted at the site of the bone injury or defect81,82,83,84. There are several ways in which hydrogels can be used as scaffolds for cell delivery. (a) Encapsulation of cells: Hydrogels can be used to encapsulate bone-forming cells, protecting them from the harsh environment at the injury site and increasing their survival. Hydrogels can also regulate the release of nutrients and growth factors, providing a controlled environment for cells to grow and proliferate85,86. (b) Promotion of cell adhesion and migration: Hydrogels that promote cell adhesion and migration can be designed by incorporating cell-adhesive peptides or other molecules into the hydrogel structure. These molecules can help to increase cell attachment and spreading within the hydrogel, leading to more effective tissue regeneration87,88,89. (c) Control of cell differentiation: Hydrogels that control the differentiation of bone-forming cells can also be designed by incorporating growth factors or other signaling molecules into the hydrogel structure. This type of hydrogel can promote the differentiation of cells into bone-forming cells, leading to more effective bone regeneration90. (d) Combination with other biomaterials: Hydrogels can be combined with other biomaterials, such as ceramics or metals, to create composite scaffolds with improved mechanical properties and bioactivity. Combining hydrogels with other biomaterials can also help promote bone regeneration by providing a favorable environment for bone-forming cells to grow and proliferate91,92,93.
Overall, hydrogels offer a versatile platform for cell delivery in bone repair by providing a 3D environment that supports cell growth and proliferation. The ability of hydrogels or their composites to encapsulate cells, promote cell adhesion and migration, and control cell differentiation makes hydrogels a promising vehicle for improving bone regeneration in various clinical situations. For instance, Chen et al. developed a porous hydrogel based on calcium silicate (CaSiO3) incorporating human umbilical vein endothelial cells (HUVECs) and Wharton’s jelly mesenchymal stem cells (WJMSCs), named PMGH94. The composite scaffold not only significantly enhanced cell proliferation and viability but also elevated the levels of angiogenic markers and bone formation proteins. Therefore, this method is believed to be highly effective for regenerating complex bone defects in deep areas.
Drug delivery systems
Hydrogels can also be used as drug delivery systems for bone repair, providing sustained release of therapeutic agents to the injury site95. There are several ways in which hydrogels can be used for drug delivery in bone repair: (a) Incorporation of drugs into the hydrogel: Drugs or other therapeutic agents can be incorporated into the hydrogel structure and then released from the hydrogel over time, sustaining the release of the therapeutic agent at the injury site. This approach can help increase the efficacy of the drug and reduce the risk of side effects96,97,98. (b) Targeting of specific cells or tissues: Hydrogels can be designed to target specific cells or tissues by incorporating cell-targeting molecules or tissue-specific ligands into the hydrogel structure. This design can help increase the drug’s localization to the injury site, leading to more effective therapy99. (c) Response to environmental stimuli: Hydrogels can be designed to respond to environmental stimuli, such as changes in pH or temperature, to release a drug in a controlled manner. This type of hydrogel can help increase the efficacy of drugs by releasing them in response to specific environmental cues100,101,102.
Hence, hydrogels offer a versatile platform for drug delivery in bone repair, providing sustained release of therapeutic agents to the injured site. As shown in Fig. 3, Wang et al. developed a thermosensitive hydrogel based on mesoporous silica nanoparticles (MSNs), which was utilized for the controlled release of stromal cell-derived factor-1 (SDF-1) and metformin to promote bone regeneration in diabetic patients. The proposed strategy involves scavenging the reactive oxygen species (ROS) generated by the disrupted glucose metabolism, thereby facilitating the recruitment of bone marrow mesenchymal stem cells (BMSCs) to promote osteogenesis103. In conclusion, the ability to incorporate drugs into hydrogels, introduce targeting to specific cells or tissues, and design hydrogels to respond to environmental stimuli makes hydrogels promising vehicles for improving bone regeneration in a variety of clinical situations. It should also be noted that improper drug loading may result in rapid release due to the high water content and porous nanostructure of hydrogels. The search for an appropriate strategy has thus emerged as a prominent and urgent research topic.
Schematic illustration of the design principles and drug delivery mechanisms of hydrogels. Reproduced with permission103. Copyright 2023, Elsevier.
Tissue engineering
Tissue engineering is a field of research that focuses on creating new tissues and organs to replace or repair damaged or diseased tissues. In bone repair, tissue engineering approaches aim to create new bone tissue using a combination of cells, biomaterials, and signaling molecules104,105,106,107. Tissue engineering can be used in bone repair in several ways. (a) Cell-based therapies: Cell-based therapies involve the use of bone-forming cells, such as MSCs, to promote bone regeneration. MSCs can be isolated from a patient’s own bone marrow or adipose tissue and then expanded in the laboratory before transplantation back into the patient at the site of the bone injury. MSCs have been shown to promote bone formation and remodeling and can also differentiate into other cell types, such as cartilage and fat cells108,109,110,111,112,113,114,115. (b) Biomaterials: Biomaterials can be used to provide a scaffold for cell growth to promote bone regeneration. Synthetic biomaterials, such as polycaprolactone (PCL) and poly(lactic-co-glycolic acid) (PLGA), can be used to create 3D scaffolds that mimic the structure of natural bone tissue. These scaffolds can then be seeded with bone-forming cells and implanted at the site of bone injury to promote bone regeneration116,117,118,119,120. (c) Signaling molecules: Signaling molecules, such as growth factors and cytokines, can be used to promote bone regeneration by stimulating the growth and differentiation of bone-forming cells121. For example, bone morphogenetic protein-2 (BMP-2) has been shown to stimulate bone formation and is commonly used in bone tissue engineering. (d) Combination therapies: Tissue engineering approaches can be combined with multiple other therapies to promote bone regeneration. A scaffold can be seeded with bone-forming cells and then treated with growth factors to promote cell differentiation and bone formation. Alternatively, a scaffold can be precoated with signaling molecules that can promote bone formation and then seeded with bone-forming cells122,123.
In brief, tissue engineering approaches offer a promising strategy for bone repair, with the potential to create new bone tissue that is biocompatible and structurally similar to natural bone tissue. Zheng et al. developed a biocompatible hydrogel composite scaffold by combining natural silk, organic sodium alginate (SA) and inorganic calcium silicate (CS). The resulting hydrogel scaffolds strongly stimulated the proliferation of BMSCs, which can be effectively applied in tissue engineering applications124. Ongoing research in tissue engineering is focused on increasing the efficiency and safety of these approaches, with the ultimate goal of developing effective bone repair therapies for use in a clinical setting.
Bone substitutes
Bone substitutes are biomaterials that can be used to replace or supplement bone tissue when the body is unable to heal itself or to aid in bone regeneration. They can be used in a variety of applications, including the repair of bone defects resulting from trauma, disease, or surgical intervention125,126. There are several types of inorganic and organic bone substitutes. (a) Ceramics: Ceramic bone substitutes, such as calcium phosphate and hydroxyapatite, are biocompatible and can stimulate bone growth. They are often used in dental implants, as well as in the repair of bone defects. (b) Polymers: Polymer bone substitutes, such as PCL, PLGA and some hydrogels, can serve as scaffolds for cell growth and bone regeneration. They can also be used to release growth factors or other signaling molecules to promote bone healing. (c) Metals: Metal bone substitutes, such as titanium and its alloys, are commonly used in orthopedic applications. These materials are solid and durable and can be designed to closely match the mechanical properties of natural bone tissue. (d) Composite materials: In composite bone substitutes, two or more materials, such as ceramics and polymers, are combined to achieve the desired properties for bone repair. For example, a composite material may be designed both to provide mechanical support and to promote bone growth.
Bone substitutes can also be categorized as either synthetic or natural matrices. Synthetic bone substitutes are usually made from materials such as ceramics, polymers, or metals, while natural bone substitutes are derived from biological sources such as human or animal tissue. Morais et al. synthesized injectable hydrogel bone substitutes by combining Alg, chitosan, and hyaluronate (HA) with glass-reinforced hydroxyapatite (GR-HAP). At pH 7.4, the Alg/HA hydrogel exhibited 80% degradation, which lasted for three days. The results indicated that all the hydrogels exhibited non-Newtonian viscoelastic fluid characteristics with low maximum extrusion forces, making them suitable as carriers for bone substitutes. Although the use of bone substitutes is a promising approach for bone repair, these approaches are not without limitations127,128,129,130,131. For example, they may not be able to mimic the complex structure and mechanical properties of natural bone tissue. Additionally, there is a risk of immune response or rejection if the material is not biocompatible or is derived from an animal source. Ongoing research in this area has focused on improving the properties of bone substitutes and developing more effective strategies for bone repair.
Implant coatings
Implant coatings are thin layers of material that are applied to the surface of an implant to enhance its biocompatibility and promote tissue integration132. Coatings can be applied via a variety of methods, including physical vapor deposition (PVD), plasma spraying, electrochemical deposition, and sol-gel coating. Some examples of implant coatings are as follows. (a) Hydroxyapatite (HA): HA is a mineral component of bone that is commonly used as a coating material for orthopedic implants. HA coatings can promote bone ingrowth and integration with the surrounding tissue133. (b) Titanium nitride (TiN): TiN is a complex, wear-resistant coating that can be used on orthopedic implants to improve their durability and resistance to wear134. (c) Diamond-like carbon (DLC): DLC is a carbon-based coating that can be used on orthopedic implants to reduce wear and friction. DLC coatings can also increase the biocompatibility of implants by reducing the likelihood of inflammation and rejection135. (d) Antibiotic coatings: Implants can be coated with antibiotics to reduce the risk of infection after surgery. Antibiotic coatings (e.g., hydrogels) can be applied using a variety of methods, including PVD and electrochemical deposition136.
Implant coatings can increase the long-term success of implants by promoting tissue integration and reducing complications such as wear, inflammation, and infection137,138,139. However, there are challenges associated with implant coatings, such as ensuring that the coating adheres securely to the implant surface and avoiding the risk of the coating delaminating or flaking off over time. Ongoing research in this area is focused on developing more effective and durable implant coatings to increase the success of orthopedic implants140,141. Hydrogels have great potential as coatings in the field of bone repair due to their unique properties and versatility142. As shown in Fig. 4, antibacterial hydrogels have become increasingly popular for antimicrobial and orthopedic applications. To date, a wide range of anti-infective hydrogels have been developed and utilized for orthopedic implants, encompassing natural matrices such as hyaluronic acid (HA), synthetic matrices such as polyethylene glycol (PEG), and composites such as HA + polylactic acid (PLA). These materials possess distinct properties that can effectively combat implant-associated infections. However, additional research is needed to fully explore the effectiveness and safety of these agents in clinical applications.
Reproduced with permission142. Copyright 2021, American Chemistry Society.
Summary
Overall, hydrogels have attracted substantial attention for bone repair and bone regeneration. Due to their 3D network structure and their advantageous mechanical and biological properties, hydrogels can be utilized as bone substitutes and in tissue engineering applications. Additionally, hydrogels can serve as versatile platforms for cell and drug delivery systems, enabling controlled release in response to environmental stimuli during therapeutic processes. Therefore, the precise release of bone stem cells, drugs, and antimicrobial agents supports cell growth and proliferation, promoting osteogenesis and regeneration in various clinical scenarios. The use of hydrogels as implant coatings can effectively reduce bacterial adhesion and increase long-term durability. The aforementioned studies support the potential of hydrogels as promising vehicles for enhancing bone repair and regeneration. All of these functions of hydrogels in bone repair and the underlying mechanisms are summarized in Table 2.
Construction strategy of hydrogels for bone repair
Semi-interpenetrating network hydrogels (semi-IPNs)
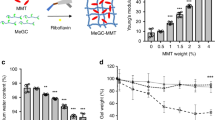
In semi-IPNs, specific polymer-based networks are crosslinked to other polymer chains, resulting in improved physicochemical properties, such as biocompatibility, mechanical strength and suitable rheological properties. For instance, Park et al. reported a semi-IPN structure containing cellulose nanofibers (CNFs) with a high aspect ratio of 240 and a polyacrylamide (PAM) mesh. Due to the strong interaction between the PAM network and rigid natural nanofibers, the compression strength of this semi-IPN system up to 3-fold higher than that of pure PAM143. Intriguingly, the PAM/BCNF composite hydrogels can be utilized for mechanical stress-responsive drug release and delivery. Similarly, this kind of hydrogel has shown great potential in the field of bone repair. For instance, Cui et al. designed semi-IPNs (OSA/Gel/CNF) through a facile one-step reaction of oxidized alginate (OSA), cellulose nanofibers, and gelatin (Gel), which mainly relied on the cooperative effects of hydrogen bonds and imine bonds32. As shown in detail in Fig. 5, during the synthesis of OSA/Gel/CNF from its three components, OSA acted as a natural reagent to link Gel via Schiff base reactions between amino and aldehyde groups, whereas CNFs formed hydrogen-bond interactions. The advantages of these semi-IPNs are as follows: (1) Compared to SA, OSA is more readily degraded in vivo and is more suitable for cell encapsulation. (2) The semi-IPNs exhibited both excellent mechanical properties and a high compressive modulus (>361.3 KPa). (3) As a result, the semi-IPNs showed good injectability and self-healing ability, both of which are promising for bone repair applications.
Reproduced with permission32. Copyright 2023, Elsevier.
Interpenetrating network hydrogels (IPNs)
The design of IPNs, which consist of intertwining double hydrogel networks in a single system during gelation, is considered another effective strategy for bone repair. IPNs can synergistically present the advantages of both types of network, including biocompatibility and high mechanical strength. As shown in Fig. 6a, Macdougall et al. synthesized flexible poly(ethylene glycol)-only (PEG) interpenetrating meshes by the addition of unfunctionalized polysaccharides144. Here, the PEG-based hydrogels were first obtained in phosphate-buffered saline (PBS) through a reaction between the alkyne and thiol end groups (nucleophilic thiol-yne click reaction). Simultaneously, some unfunctionalized natural polymers (e.g., chitosan, gelatin, heparin, alginate, HA, etc.) were incorporated to form a secondary loose crosslinked network driven by electrostatic forces. This strategy endows the crosslinked IPNs with good stretchability and enhanced tensile performance (Fig. 6b), as well as self-healing capabilities (Fig. 6c). Therefore, IPN materials possess the advantages of both of their component systems and can effectively overcome the disadvantages associated with each network. Consequently, these materials have potential for further application in bone repair.
a Synthetic procedure for PEG-based hydrogels. b Measurements of tensile stress/strain and self-healing properties. Reproduced with permission144. Copyright 2018, Royal Society of Chemistry.
Hybrid hydrogels
Bioactive glass composite hydrogels
The periosteum is known to play key roles in mineralization, vascularization and protection during bone tissue regeneration145. However, many existing artificial periosteal grafts focus only on protection and lack the functions of osteogenesis and angiogenesis. Xin et al. developed a novel inorganic reinforced gelatin hydrogel membrane as an artificial periosteum to enhance durable angiogenesis and osteogenesis in bone reconstruction using inorganic and organic co-crosslinked dual networks146. As shown in Fig. 7a, mesoporous bioactive glass nanoparticles (MBGNs) were incorporated into a photo-crosslinked gelatin methacrylate (GelMA) hydrogel, affording a double-network structure with increased structural stability and improved mechanical properties (Fig. 7b). A GelMA-G-MBGN membrane enabled osteogenic differentiation both in vivo and in vitro, which is an important factor for cell differentiation and proliferation. In general, hydrogel membranes, characterized by prolonged degradation time, pH stability, biomineralization capability, and sustained ion release over an extended period of time, represent a promising approach for the development of advanced periosteal biomaterials with superior tactile sensation and bone regeneration properties.
a Inorganic strengthened hydrogel membrane for regenerative periosteum. b Fabricating amino modified MBGNs and GelMA-MBGNs (G-MBGNs). Reproduced with permission146. Copyright 2017, American Chemistry Society.
Cell- or protein-grafted hydrogels
Xin et al. further grafted recombinant human bone morphogenetic protein-2 (rhBMP-2) onto the surface of MBGNs via amide bonds and performed photocrosslinking with GelMA147. As shown in Fig. 8, GelMA/MBGNS-rhBMP-2 hydrogel membranes were prepared to release RhBMP-2 in a controlled manner during the early stage of bone regeneration, followed by the release of calcium and silica ions for the long-term promotion of osteogenesis. The early release of rhBMP-2 can effectively promote local cell osteogenic differentiation in a short time. Inorganic ions can not only promote cell adhesion in the early stage but also continuously promote osteogenic differentiation in the long term. Moreover, the GelMA/MBGN-rhBMP-2 hydrogel showed excellent long-term promotion of osteogenesis and bone tissue regeneration in critical-size skull defects in rats. This presents a possible way to use bioactive factors such as rhBMP-2 in a more controlled and safe manner to accelerate bone repair.
It shows excellent long-term osteogenesis and the ability to promote bone tissue regeneration in critical-size skull defects in rats. Reproduced with permission147. Copyright 2018, American Chemistry Society.
In addition, Chen’s group used 3D printing technology to fabricate polyamine-modified calcium silicate (PDACS)/polycaprolactone (PCL) scaffolds by combining WJMSCs with HUVEC-loaded hydrogels (Fig. 9a)94. This mixture of synthetic gel materials with cellular components not only promoted osteogenesis but also stimulated angiogenesis, leading to the development of vascular networks, which suggests that solvent-free 3D printing can be further applied to improve many aspects of bone tissue regeneration (Fig. 9b)94. In summary, this innovative strategy of integrating cells or proteins into hydrogels holds immense potential for the regeneration of intricate hard tissues to repair defects in deep bone structures.
The HUVECs were encapsulated in hydrogels and dispensed into the pores, and WJMSCs were placed on the PDASC/PCL scaffold via a piezoelectric needle. a Schematic diagram of the bioprinting process. b Schematic diagram of PM, PMG, PGH, and PMGH groups. Reproduced with permission94. Copyright 2018, Elsevier.
CaCO3-based bioceramics and hydrogels
The utilization of composite hydrogels in bone regeneration is limited due to their inadequate ossification performance. To address this issue, a new osteoblastic gellin glue gel was designed by Abalymov et al. As shown in Fig. 10a, a calcium carbonate (CaCO3)-based composite bioceramic and hydrogel were generated and used to encapsulate and immobilize the enzyme148. The addition of CaCO3 to the hydrogel increased the stability of the particles and decreased the enzyme activity. The CaCO3-incorporated alginate hydrogels remained stable for almost 13 days, much longer than calcium alginate-based hydrogels and Alg-based hydrogels (Fig. 10b). In addition, the CaCO3-based hydrogels showed high protein loading capacity, reaching 37% by weight. According to the results of cell experiments, the composite hydrogels containing alkaline phosphatase (ALP) resulted in a higher concentration of hydroxyapatite, suggesting their potential in bone repair treatment. These results indicated that composite hydrogels containing Ca/Mg CaCO3 submicron particles effectively optimized bone remodeling and other cell growth applications. Therefore, hydrogels can serve as highly efficient and biocompatible carriers for drug delivery in bone reconstruction applications.
It illustrates that the gel was obtained via thermal annealing and the insertion of Ca/Mg particles. a Schematic of experiment. b Graphical abstract and calcein AM (green), osteoimage mineralization assay (Cyan) and hydroxyapatite deposition measurement at different times on MC3T3-E1 cells. Reproduced with permission148. Copyright 2020, American Chemistry Society.
2D black phosphorus nanosheet-based composite hydrogels
Many challenges in bone tissue engineering need to be solved to achieve efficient bone regeneration. To enhance stem cell function and generate a vascularized network, a novel strategy was proposed: to accelerate bone regeneration by continuously providing phosphorus (P) rather than exogenous calcium (Ca). Huang’s group developed a hydrogel platform based on two-dimensional black phosphorus nanosheets (BPNs), which can provide slow and sustained release of P149. Hydrogels were prepared by the photoinduced crosslinking of GelMA, BPNs, and cationic arginine-based unsaturated polyester amides. The incorporated BPNs were shown to be encapsulated inside the hydrogel scaffold (Fig. 11), which acted as ECM for bone reconstruction. Moreover, the introduction of BPNs helped to improve the mechanical properties of the hydrogels, which can also release P in response to light and accelerate mineralization in vitro. In vivo results in a rabbit bone defect model confirmed the biocompatibility of the BPNs and showed that the BPNs helped to accelerate bone regeneration. In addition, the addition of BPNs promoted the osteogenic differentiation of human dental pulp stem cells (hDPSCs) via the BMP-RUNX2 pathway. These results strongly suggest that a strategy of continuously supplying Ca/P from BPN-containing hydrogel platforms is promising for efficient bone regeneration.
It supplies P rather than Ca to promote biomineralization and bone regeneration. Reproduced with permission149. Copyright 2019, American Chemistry Society.
ChS-NP-based composite hydrogels
Numerous research teams are exploring the application of hydrogels in bone regeneration through the incorporation of nanoparticle. As a representative work, Radhakrishnan’s group developed an injectable semi-IPN network hydrogel structure using chondroitin sulfate nanoparticles (ChS-NPs) and nano-HA (~30–90 nm) in the cartilage and subchondral hydrogel regions, respectively150. The results showed that the mineralized subchondral hydrogel significantly promoted osteoblast proliferation and ALP activity (p < 0.05), and the nanoengineered gradient hydrogel increased cartilage regeneration with subchondral bone formation and side-to-side host tissue integration. Therefore, composite hydrogels incorporating nanoparticles have become a simple but important tool due to their remarkable ability to promote osteogenic differentiation.
Summary
Hydrogel materials possess distinct structural characteristics and physical properties, including a 3D network structure, implantability, favorable mechanical and degradation properties, and exceptional biological effects151,152,153. Various preparation techniques, such as chemical crosslinking and physical doping, have been employed to construct hydrogel materials (i.e., semi-IPNs, IPNs and hybrid hydrogels), which hold promise for fulfilling the material requirements for the clinical treatment of bone defects. In particular, different factors, including bioactive glass, cells or proteins, inorganic matrices (e.g., CaCO3), 2D nanosheets (e.g., black phosphorus) and nanoparticles (e.g., CdS NPs), have been incorporated into conventional hydrogel systems to investigate their effects in the treatment of bone defects. Accordingly, a synergistic treatment strategy has become crucial for the development of high-performance hydrogel systems whose osteogenic properties can increase therapeutic efficacy.
Conclusion and outlook
To effectively address bone defects, various therapeutic strategies, encompassing nonsurgical approaches such as casting, electrical stimulation, and ultrasound therapy as well as surgical interventions such as internal fixation, external fixation, bone grafting, and bone regeneration, are commonly employed in clinical practice. The limitations of these traditional treatment modalities, such as the risks associated with immune rejection, donor site injury, and transmission of infectious diseases, emphasize the urgency and challenge of the development of high-performance biomaterials.
The development of high-performance and multifunctional biomaterials for the treatment of bone defects is essential. At present, three types of materials are used in bone tissue engineering: naturally derived biomaterials, synthetic biomaterials, and metal materials. The earliest application of bone tissue engineering involved mainly thermal-composite hydrogels, but the ossification performance of this method was poor, so osteogenic hydrogels were developed by thermal annealing154,155. At that time, the main goal was to study the biomechanics of embryonic cartilage. Later, scientists used a solvent-free process to combine glial mesenchymal stem cells with human umbilical vein endothelial cell (HUVEC)-loaded hydrogels to prepare polyamine-modified calcium silicate/polycaprolactone scaffolds. The gelatin was modified with methacrylic anhydride to obtain photo crosslinked methacrylic gelatin. By photocrosslinking with methacrylate gelatin, the hydrogel films were prepared to release Ca and Si ions in a controlled manner at the early stage of bone regeneration for the long-term promotion of osteogenesis156,157,158. Since then, great breakthroughs have been made in bone tissue engineering, and a tough and flexible amphoteric copolymer-based hydrogel with bioactive groups has been created for bone regeneration. In 2019, Hasani’s group developed a unique bioinspired adhesive hydrogel with tunable mechanical properties and biodegradability that has been applied in dental clinical medicine. Subsequently, Wojda et al. developed a biomaterial system for the delivery of hydrogels to significant bone defects to promote bone regeneration159.
With the ongoing development of materials science, numerous multifunctional materials have emerged and been applied in various aspects of biomedicine. In recent years, hydrogels have been considered promising candidate materials for tissue engineering and bone repair research due to their structural and compositional similarity to the ECM, high water content, satisfactory biocompatibility, and tunable biophysical and biochemical properties160,161. In addition, some hydrogels also have the advantages of low cost, multifunctionality, renewability, degradability, and excellent biocompatibility159,162,163. Therefore, the present study offers a comprehensive overview of the recent advances and challenges in the utilization of hydrogels for bone repair and regeneration. The application of hydrogels in the field of orthopedics has focused mainly on tissue engineering, wound healing, and drug delivery. Excitingly, some research results have been applied in clinical practice. A variety of hydrogel structures, including osteogenic gellan gel, semi-interpenetrating network hydrogels (semi-IPNs), interpenetrating network hydrogels (IPNs), and photoinduced crosslinking methacrylate gelatin (MAGel), have been described. The functional, mechanistic, and medical advantages of these hydrogel structures are thoroughly examined in this review. With the development of hydrogels and bone repair techniques and further understanding of the cellular signaling mechanisms involved in tissue repair, hydrogels will be able to mimic the natural ECM more accurately and play a more effective role in bone and soft tissue engineering.
According to previous reports, conventional synthetic hydrogels still present several disadvantages and challenges, such as an isotropic network structure, insufficient mechanical properties, weak tissue adhesion and lack of bone conductivity. Most hydrogels serve as carriers rather than promoters of bone healing. The development of dual-functional or multifunctional hydrogels and their composites represents an intriguing research topic. Considering the diverse structures of hydrogels, it is essential to elucidate structure-property relationships to better understand the principles and mechanisms involved in bone repair. Furthermore, research on microfabrication techniques, including 3D printing, is necessary to achieve controllable nanostructures and large-scale production164,165,166,167,168. Therefore, collaborative research efforts are still needed to enhance the performance of these materials in the field of bone healing. The development of novel and highly efficient hydrogel systems could revolutionize bone defect treatment.
References
Guo, X. Dong, X., Zou, G., Gao, H. & Zhai, W. Strong and tough fibrous hydrogels reinforced by multiscale hierarchical structures with multimechanisms. Sci. Adv. 9, eadf7075 (2023).
de Souza Balbinot, G. et al. Niobium-containing bioactive glasses modulate alkaline phosphatase activity during bone repair. J. Biomed. Mater. Res. 111, 1224 (2023).
Xu, T. et al. Accelerating the prediction and discovery of peptide hydrogels with human-in-the-loop. Nat. Commun. 14, 3880 (2023).
Sun, H. et al. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact. Mater. 24, 477 (2023).
Zhang, C. et al. Visual growth of nano-HOFs for low-power memristive spiking neuromorphic system. Nano Energy 109, 108274 (2023).
Zhang, P. et al. Dual coordination between stereochemistry and cations endows polyethylene terephthalate fabrics with diversiform antimicrobial abilities for attack and defense. ACS Appl. Mater. Interfaces 15, 9926 (2023).
Zhang, C. et al. Variable learning-memory behavior from π‐conjugated ligand to ligand-containing cobalt(II) complex. Chin. J. Chem. 40, 2296 (2022).
Zhang, C., Li, Y., Li, H., Zhang, Q. & Lu, J. Overview of electric-field-induced deposition technology in fabricating organic thin films. J. Mater. Chem. C 9, 374 (2021).
Assanah, F. et al. Ultrasound-derived mechanical stimulation of cell-laden collagen hydrogels for bone repair. J. Biomed. Mater. Res. A 111, 1200 (2023).
Zhang, X. et al. Bone marrow mesenchymal stem cells paracrine TGF-β1 to mediate the biological activity of osteoblasts in bone repair. Cytokine 164, 156139 (2023).
Gao, X. et al. Chitosan-vancomycin hydrogel incorporated bone repair scaffold based on staggered orthogonal structure: a viable dually controlled drug delivery system. RSC Adv 13, 3759 (2023).
He, C. et al. Random and aligned electrostatically spun PLLA nanofibrous membranes enhance bone repair in mouse femur midshaft defects. J. Biomater. Appl. 37, 1582 (2023).
Chang, Y. et al. Lactoferrin mediates enhanced osteogenesis of adipose-derived stem cells: Innovative molecular and cellular therapy for bone repair. Int. J. Mol. Sci. 24, 1749 (2023).
Lee, J., Byun, H., Madhurakkat Perikamana, S. K., Lee, S. & Shin, H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthc. Mater 8, 1801106 (2019).
Tiwari, J. N. et al. Accelerated bone regeneration by two-photon photoactivated carbon nitride nanosheets. ACS Nano 11, 742 (2017).
Somers, N. et al. Fabrication of doped β-tricalcium phosphate bioceramics by Direct Ink Writing for bone repair applications. J. Eur. Ceram. Soc. 43, 629 (2023).
Ebrahimi, S. et al. The efficacy of teriparatide (Cinnopar®) on bone repair in mandibular fractures: A single blinded randomized clinical trial. J. Cranio Maxill. Surg. 50, 923 (2022).
Zhang, Q. et al. High-strength hydroxyapatite scaffolds with minimal surface macrostructures for load-bearing bone regeneration. Adv. Funct. Mater. 32, 2204182 (2022).
Chacon, E. L. et al. Collagen-chitosan-hydroxyapatite composite scaffolds for bone repair in ovariectomized rats. Sci. Rep. 13, 28 (2023).
Li, W. et al. Supramolecular ionogels tougher than metals. Adv. Mater. 35, 2301383 (2023).
Zhang, Y., Dai, Y., Xia, F. & Zhang, X. Gelatin/polyacrylamide ionic conductive hydrogel with skin temperature-triggered adhesion for human motion sensing and body heat harvesting. Nano Energy 104, 107977 (2022).
Wu, H. et al. Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophages and bone repair via MAPK/JNK inhibition and OXPHOS activation. Biomaterials 293, 121990 (2023).
Costa, R. R., Freitas, R. D. S., da Cunha, G., de Oliveira, S. D. & Weber, J. B. B. Antimicrobial and bone repair effects of boric acid in a rat model of dry socket (alveolar osteitis) following dental extraction. J. Trace Elem. Med. Bio. 76, 127118 (2023).
Boschetto, F. et al. Development and evaluation of osteogenic PMMA bone cement composite incorporating curcumin for bone repairing. Mater. Today Chem. 27, 101307 (2023).
Ding, L. et al. Preparation and characterizations of an injectable and biodegradable high-strength iron-bearing brushite cement for bone repair and vertebral augmentation applications. Biomater. Sci. 11, 96 (2022).
Di, W. et al. A bifunctional zoledronate sustained-release system in scaffold: Tumor therapy and bone repair. Colloid. Surface. B 222, 113064 (2023).
Li, H. et al. Polyetheretherketone microspheres loaded with cerium dioxide nanoparticles mitigate damage from cellular oxidative stress and promote bone repair. Mater. Des. 225, 111426 (2023).
Yin, J. et al. Acceleration of bone repair in critical-size defect using angiopoietin-2 associated with novel carbon nanotubes scaffold via mitophagy-pyroptosis pathway. Eur. Rev. Med. Pharmacol. Sci. 26, 8969 (2022).
Wu, J., Wang, S., Zheng, Z. & Li, J. Fabrication of Biologically inspired electrospun Collagen/Silk fibroin/bioactive glass composited nanofibrous scaffold to accelerate the treatment efficiency of bone repair. Regen. Ther. 21, 122 (2022).
Machowska, A. et al. Clindamycin-loaded halloysite nanotubes as the antibacterial component of composite hydrogel for bone repair. Polymers 14, 5151 (2022).
Almeida, C. D. S. C., Alves, A., de Brito Resende, R. F., de Albuquerque Calasans-Maia, J. & Moraschini, V. Does melatonin associated with nanostructured calcium phosphate improve alveolar bone repair? Medicina 58, 1720 (2022).
Cui, S., Zhang, S. & Coseri, S. An injectable and self-healing cellulose nanofiber-reinforced alginate hydrogel for bone repair. Carbohydr. Polym. 300, 120243 (2023).
Nadine, S., Fernandes, I. J., Correia, C. R. & Mano, J. F. Close-to-native bone repair via tissue engineered endochondral ossification approaches. iScience 25, 105370 (2022).
Zhao, Y. et al. Porous hydroxyapatite scaffold orchestrated with bioactive coatings for rapid bone repair. Biomater. Adv 144, 213202 (2023).
Ono, N. The mechanism of bone repair: Stem cells in the periosteum dedicated to bridging a large gap. Cell Rep. Med. 3, 100807 (2022).
Chen, M. et al. ROS-activatable biomimetic interface mediates in-situ bioenergetic remodeling of osteogenic cells for osteoporotic bone repair. Biomaterials 291, 121878 (2022).
Tan, T. et al. ROS-activatable biomimetic interface mediates in-situ bioenergetic remodeling of osteogenic cells for osteoporotic bone repair. Materials 15, 7898 (2022).
Zhao, Q. & Gao, S. Poly (butylene succinate)/silicon nitride nanocomposite with optimized physicochemical properties, biocompatibility, degradability, and osteogenesis for cranial bone repair. J. Funct. Biomater. 13, 231 (2022).
Zhang, Y. et al. Thermosensitive hydrogel loaded with concentrated growth factors promote bone repair in segmental bone defects. Front. Bioeng. Biotech. 10, 1039117 (2022).
Li, B. M., Yang, H., Shu, Y., Xiao, W. & Zhu, S. Biomimetic mineralization of poly (L-lactic acid) nanofibrous microspheres for bone regeneration. Mater. Today Commun. 33, 104682 (2022).
Mussatto, A. et al. High strength bioinspired calcium phosphate-based material for bone repair applications. Mater. Today Commun. 33, 104693 (2022).
Marcucio, R. S., Miclau, T. C. & Bahney, S. A shifting paradigm: Transformation of cartilage to bone during bone repair. J. Dent. Res. 102, 13 (2022).
Zhang, H. et al. Expert consensus on the bone repair strategy for osteoporotic fractures in China. Front. Endocrinol. 13, 989648 (2022).
Alarçin, E., Dokgöz, A. B., Akgüner, Z. P., Seki, H. & Bal-Öztürk, K. A. Eggshell integrated GelMA/CSMA/HyMA hybrid hydrogels for cell therapy/tissue engineering. J. Drug Deliv. Sci. Tec. 77, 103844 (2022).
Zhou, X. et al. nHA-loaded gelatin/alginate hydrogel with combined physical and bioactive features for maxillofacial bone repair. Carbohydr. Polym. 298, 120127 (2022).
Wang, Y., Zhang, H., Hu, Y., Jing, Y. & Geng, Z. Bone repair biomaterials: a perspective from immunomodulation. Adv. Funct. Mater. 32, 2208639 (2022).
Naveda, R. Dos et al. Midpalatal suture bone repair after miniscrew-assisted rapid palatal expansion in adults. Prog. Orthod. 23, 35 (2022).
Hou, X. et al. Calcium phosphate-based biomaterials for bone repair. J. Funct. Biomater. 13, 187 (2022).
Xia, Y., Xu, W., Zhang, H., Wu, X. & Dai, H. 3D-printing polylactic acid/hydroxyapatite fracture internal fixation plates for bone repair. J. Appl. Polym. Sci. 139, e53147 (2022).
Pomini, K. T. et al. Use of photobiomodulation combined with fibrin sealant and bone substitute improving the bone repair of critical defects. Polymers 14, 4170 (2022).
Felice, P. et al. Reverse guided bone regeneration (R-GBR) digital workflow for atrophic jaws rehabilitation. Appl. Sci. 12, 9947 (2022).
Takafuji, Y. et al. Extracellular vesicles secreted from mouse muscle cells improve delayed bone repair in diabetic mice. Endocr. J. 70, 161 (2023).
Song, L. Effects of exercise or mechanical stimulation on bone development and bone repair. Stem Cells Int 2022, 5372229 (2022).
Miao, M. et al. The miRNA-144-5p/IRS1/AKT axis regulates the migration, proliferation, and mineralization of osteoblasts: A mechanism of bone repair in diabetic osteoporosis. Cell Biol. Int. 46, 2220 (2022).
Yang, C. et al. New dual-function in situ bone repair scaffolds promote osteogenesis and reduce infection. J. Biol. Eng. 16, 23 (2022).
Chen, M. et al. A self-healing, magnetic and injectable biopolymer hydrogel generated by dual cross-linking for drug delivery and bone repair. Acta Biomater. 153, 159 (2022).
Zeng, Y. et al. Injectable temperature-sensitive hydrogel system incorporating deferoxamine-loaded microspheres promotes H-type blood vessel-related bone repair of a critical size femoral defect. Acta Biomater. 153, 108 (2022).
Chang, S. et al. A sustained release of alendronate from an injectable tetra-PEG hydrogel for efficient bone repair. Front. Bioeng. Biotech. 10, 961227 (2022).
Liu, X. et al. Hydrophilic competent and enhanced wet-bond strength castor oil-based bioadhesive for bone repair. Colloid. Surface B 219, 112835 (2022).
Yang, C. et al. Single-cell spatiotemporal analysis reveals cell fates and functions of transplanted mesenchymal stromal cells during bone repair. Stem Cell Rep. 17, 2318 (2022).
Meng, Z. et al. N-acetylcysteine regulates dental follicle stem cell osteogenesis and alveolar bone repair via ROS scavenging. Stem Cell Res. Ther. 13, 466 (2022).
Zhang, Z., Yang, X., Cao, X., Qin, A. & Zhao, J. Current applications of adipose-derived mesenchymal stem cells in bone repair and regeneration: A review of cell experiments, animal models, and clinical trials. Front. Bioeng. Biotech. 10, 942128 (2022).
Tian, B. et al. A 3D-printed molybdenum-containing scaffold exerts dual pro-osteogenic and anti-osteoclastogenic effects to facilitate alveolar bone repair. Int. J. Oral Sci. 14, 45 (2022).
Kato, D. et al. Gain-of-function of FGFR3 accelerates bone repair following ischemic osteonecrosis in juvenile mice. Calcified Tissue Int. 111, 622 (2022).
Chen, H. et al. A new injectable quick hardening anti-collapse bone cement allows for improving biodegradation and bone repair. Biomater. Adv. 141, 213098 (2022).
Grecula, M. J. Impact of octacalcium phosphate/gelatin (OCP/Gel) composite on bone repair in refractory bone defects Clin. Orthop. Relat. R. 480, 2043 (2022).
Moncal, K. K. et al. Controlled Co-delivery of pPDGF-B and pBMP-2 from intraoperatively bioprinted bone constructs improves the repair of calvarial defects in rats. Biomaterials 281, 121333 (2022).
Han, C. et al. Quercetin-loaded nanocomposite microspheres for chronologically promoting bone repair via synergistic immunoregulation and osteogenesis. Mater. Design 222, 111045 (2022).
Liu, Z. et al. Self-assembled terbium-amino acid nanoparticles as a model for terbium biosafety and bone repair ability assessment. Compos. Part B Eng 244, 110186 (2022).
Wang, L., Zeng, X., Chen, X., Zeng, X. & Luo, K. Programmable, biodegradable composite scaffolds with variable pore morphology for minimal invasive bone repair. Compos. Part A Appl. S 162, 107130 (2022).
Chu, W. et al. Dual crosslinking hydrogels with tunable injectability and stability for bone repair. J. Mater. Chem. B 10, 6237 (2022).
Tabanez, A. P. et al. FTY720 administration results in a M2 associated immunoregulatory effect that positively influences the outcome of alveolar bone repair outcome in mice. Bone 163, 116506 (2022).
Sharifi, M. et al. Criteria, challenges, and opportunities for acellularized allogeneic/xenogeneic bone grafts in bone repairing. ACS Biomater. Sci. Eng. 8, 3199 (2022).
Xu, Z. Research trends and progress in the field of metal materials and bone repair: Comprehensive bibliometric and visual analysis (2012-2021). Front. Mater. 9, 954525 (2022).
Kinoshita, Y. et al. Irisin improves delayed bone repair in diabetic female mice. J. Bone Miner. Metab. 40, 735 (2022).
Oztekin, F. et al. In vivo evaluation of the effects of B-doped strontium apatite nanoparticles produced by hydrothermal method on bone repair. J. Funct. Biomater. 13, 110 (2022).
Zhang, S. et al. Tissue engineered bone via templated hBMSCs mineralization and its application for bone repairing. Biomater. Adv. 139, 212937 (2022).
Wachol, K. et al. Comparative analysis of implant prosthesis treatment planning and execution following bone repair procedures using dynamic surgical navigation in augmented areas. Coatings 12, 1099 (2022).
Swain, S. et al. Microstructural, dielectric, mechanical, and biological properties of hydroxyapatite (HAp)/BZT-BCT (0.5Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3)TiO3) bio-composites with improved mechano-electrical properties for bone repair. Ceram. Int. 48, 24505 (2022).
Hu, X. et al. Recent progress in 3D printing degradable polylactic acid-based bone repair scaffold for the application of cancellous bone defect. MedComm-Biomater. Appl 1, e14 (2022).
Wang, C. et al. Polylactic acid scaffold with directional porous structure for large-segment bone repair. Int. J. Biol. Macromol. 216, 810 (2022).
Yu, L. et al. Carboxymethyl chitosan-alginate enhances bone repair effects of magnesium phosphate bone cement by activating the FAK-Wnt pathway. Bioact. Mater. 20, 598 (2023).
Tan, Y. et al. Injectable hyaluronic acid/hydroxyapatite composite hydrogels as cell carriers for bone repair. Int. J. Biol. Macromol. 216, 547 (2022).
Xu, Y. et al. Immunology and bioinformatics analysis of injectable organic/inorganic microfluidic microspheres for promoting bone repair. Biomaterials 288, 121685 (2022).
Qi, D. et al. Application of porous polyetheretherketone scaffold/vancomycin-loaded thermosensitive hydrogel composites for antibacterial therapy in bone repair. Macromol. Biosci. 22, 2200114 (2022).
Yao, R. et al. Fabrication and characterization of biodegradable Zn scaffold by vacuum heating-press sintering for bone repair. Biomater. Adv. 138, 212968 (2022).
Liu, J.-Q. et al. Current status and prospects of metal-organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 10, 5105 (2022).
Wu, Q. et al. Modification of adipose mesenchymal stem cells-derived small extracellular vesicles with fibrin-targeting peptide CREKA for enhanced bone repair. Biomater. Adv. 20, 208 (2023).
Kubaszek, B. et al. Radiological and microbiological evaluation of the efficacy of alveolar bone repair using autogenous dentin matrix-preliminary study. Coatings 12, 909 (2022).
Liang, H. et al. Trabecular-like Ti-6Al-4V scaffold for bone repair: A diversified mechanical stimulation environment for bone regeneration. Compos. Part B Eng 241, 110057 (2022).
Hu, Z. et al. Engineering BPQDs/PLGA nanospheres-integrated wood hydrogel bionic scaffold for combinatory bone repair and osteolytic tumor therapy. Chem. Eng. J. 446, 137269 (2022).
Youness, R. A., Ibrahim, M. A. & Taha, M. A. Evaluation of the electrical and dielectric behavior of the apatite layer formed on the surface of hydroxyapatite/hardystonite/copper oxide hybrid nanocomposites for bone repair applications. Ceram. Int. 48, 19837 (2022).
Wei, P. et al. Synthesis and properties of high performance biobased liquid crystal copolyesters toward load-bearing bone repair application. Eur. Polym. J. 173, 111278 (2022).
Chen, Y.-W. et al. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 91, 679 (2018).
Chen, Y. et al. A composite of cubic calcium-magnesium sulfate and bioglass for bone repair. Front. Bioeng. Biotechnol. 10, 898951 (2022).
Ahmadipour, S., Varshosaz, J., Hashemibeni, B., Manshaei, M. & Safaeian, L. In vivo assessment of bone repair by an injectable nanocomposite scaffold for local co-delivery of autologous platelet-rich plasma and calcitonin in a rat model. Drug Dev. Ind. Pharm. 48, 98 (2022).
Hamada, S. et al. Octacalcium phosphate/gelatin composite (OCP/Gel) enhances bone repair in a critical-sized transcortical femoral defect rat model. Clin. Orthop. Relat. R 480, 2043 (2022).
Nakayama, M. et al. Single-cell RNA sequencing unravels heterogeneity of skeletal progenitors and cell-cell interactions underlying the bone repair process. Regen. Ther 21, 9 (2022).
Negut, I. et al. Implant surfaces containing bioglasses and ciprofloxacin as platforms for bone repair and improved resistance to microbial colonization. Pharmaceutics 14, 1175 (2022).
Huang, Y. et al. A non-invasive smart scaffold for bone repair and monitoring. Bioact. Mater. 19, 499 (2023).
Chu, W. et al. Dual crosslinking hydrogels with tunable injectability and stability for bone repair. J. Mater. Chem. B 10, 4386 (2022).
Basanth, A., Mayilswamy, N. & Kandasubramanian, B. Bone regeneration by biodegradable polymers. Polym. Plast. Technol. Mater. 61, 816 (2022).
Wang, H. et al. Bioinspired drug-delivery system emulating the natural bone healing cascade for diabetic periodontal bone regeneration. Bioact. Mater. 21, 324 (2023).
Zhang, J. et al. Mechanical loading attenuated negative effects of nucleotide analogue reverse-transcriptase inhibitor TDF on bone repair via Wnt/β-catenin pathway. Bone 161, 116449 (2022).
Reis, C. H. B. et al. Effects of a biocomplex formed by two scaffold biomaterials, hydroxyapatite/tricalcium phosphate ceramic and fibrin biopolymer, with photobiomodulation, on bone repair. Polymers 14, 2075 (2022).
Johanson, Z., Liston, J., Davesne, D., Challands, T. & Meredith Smith, M. Mechanisms of dermal bone repair after predatory attack in the giant stem-group teleost Leedsichthys problematicus Woodward, 1889a (Pachycormiformes). J. Anat. 241, 393 (2022).
Peng, Y. et al. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact. Mater 6, 3109 (2021).
Rapalli, V. K., Sharma, S., Roy, A., Alexander, A. & Singhvi, G. Solid lipid nanocarriers embedded hydrogel for topical delivery of apremilast: In-vitro, ex-vivo, dermatopharmacokinetic and anti-psoriatic evaluation. J. Drug Deliv. Sci. Tech. 63, 102442 (2021).
Li, Y. et al. Copper oxide functionalized chitosan hybrid hydrogels for highly efficient photocatalytic-reforming of biomass-based monosaccharides to lactic acid. Appl. Catal. B Environ. 291, 120123 (2021).
Yang, J. et al. Advanced applications of chitosan-based hydrogels: From biosensors to intelligent food packaging system. Trends Food Sci. Tech. 110, 822 (2021).
Su, Z., Li, Y., Li, J. & Dou, X. Ultrasensitive luminescent turn-on detection of perchlorate particulates by triggering supramolecular self-assembly of platinum (II) complex in hydrogel matrix. Sensor. Actuat. B Chem 336, 129728 (2021).
Zhang, Y., Luo, Q., Ding, K., Liu, S. G. & Shi, X. A smartphone-integrated colorimetric sensor of total volatile basic nitrogen (TVB-N) based on Au@ MnO2 core-shell nanocomposites incorporated into hydrogel and its application in fish spoilage monitoring. Sensor. Actuat. B Chem. 335, 129708 (2021).
Dai, L. et al. A green all-polysaccharide hydrogel platform for sensing and electricity harvesting/storage. J. Power Sources 493, 229711 (2021).
Abou Taleb, M. F., Abou El Fadl, F. I. & Albalwi, H. Adsorption of toxic dye in wastewater onto magnetic NVP/CS nanocomposite hydrogels synthesized using gamma radiation. Sep. Purif. Technol. 266, 118551 (2021).
Helú, M. A. & Liu, L. Rational shaping of hydrogel by electrodeposition under fluid mechanics for electrochemical writing on complex shaped surfaces at microscale. Chem. Eng. J. 416, 129029 (2021).
Bashir, S., Hina, M., Ramesh, S. & Ramesh, K. Flexible and self-healable poly (N, N-dimethylacrylamide) hydrogels for supercapacitor prototype. Colloid. Surface. A 617, 126377 (2021).
Goliszek, M., Kołodyńska, D., Pylypchuk, I. V., Sevastyanova, O. & Podkościelna, B. Synthesis of lignin-containing polymer hydrogels with tunable properties and their application in sorption of nickel (II) ions. Ind. Crop. Prod. 164, 113354 (2021).
Xiang, S.-L., Su, Y.-X., Yin, H., Li, C. & Zhu, M.-Q. Visible-light-driven isotropic hydrogels as anisotropic underwater actuators. Nano Energy 85, 105965 (2021).
Lan, W. et al. Physicochemical properties and biocompatibility of the bi-layer polyvinyl alcohol-based hydrogel for osteochondral tissue engineering. Mater. Des. 204, 109652 (2021).
Yoshida, A. & Tsujimura, S. Improved glucose oxidation catalytic current generation by an FAD-dependent glucose dehydrogenase-modified hydrogel electrode, in accordance with the Hofmeister effect. J. Phys. Energy 3, 024005 (2021).
Zhu, P., Huang, W., Guo, X. & Chen, L. Strong and elastic pea protein hydrogels formed through pH-shifting method. Food Hydrocolloid 117, 106705 (2021).
Ding, J. et al. Chitosan hydrogel derived carbon foam with typical transition-metal catalysts for efficient water splitting. Carbon 177, 160 (2021).
Groult, S., Buwalda, S. & Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 149, 110386 (2021).
Zheng, A. et al. Biocompatible silk/calcium silicate/sodium alginate composite scaffolds for bone tissue engineering. Carbohydr. Polym. 199, 244–255 (2018).
Feng, P. et al. A dual stimuli-responsive and safer controlled release platform of pesticide through constructing UiO-66-based alginate hydrogel. Polym. Testing 97, 107152 (2021).
El-saied, H. A.-a & El-Fawal, E. M. Green superabsorbent nanocomposite hydrogels for high-efficiency adsorption and photo-degradation/reduction of toxic pollutants from waste water. Polym. Testing 97, 107134 (2021).
Morais, D. S. et al. Development and characterization of novel alginate-based hydrogels as vehicles for bone substitutes. Carbohydr. Polym. 95, 134–142 (2013).
Klongklaew, P. & Bunkoed, O. The enrichment and extraction of parabens with polydopamine-coated microporous carrageenan hydrogel beads incorporating a hierarchical composite of metal-organic frameworks and magnetite nanoparticles. Microchem. J. 165, 106103 (2021).
Liu, G., Cui, C., Jiang, L., Gao, H. & Gao, J. Visible light-induced hydrogels towards reversible adsorption and desorption based on trivalent chromium in aqueous solution. React. Funct. Polym. 163, 104886 (2021).
Li, H. et al. Gellan gum hydrogel as an aqueous treatment method for xuan paper. Restaurator 42, 37 (2021).
Rud, O. V., Landsgesell, Holm, J. C. & Košovan, P. Modeling of weak polyelectrolyte hydrogels under compression-Implications for water desalination. Desalination 506, 114995 (2021).
Xiao, S. et al. Strong anti-polyelectrolyte zwitterionic hydrogels with superior self-recovery, tunable surface friction, conductivity, and antifreezing properties. Eur. Polym. J 148, 110350 (2021).
Bose, B., Davis, C. A. & Erk, R. K. Microstructural refinement of cement paste internally cured by polyacrylamide composite hydrogel particles containing silica fume and nanosilica. Cement Concr. Res. 143, 106400 (2021).
Habib, A. & Khoda, B. Fiber-filled hybrid hydrogel for bio-manufacturing. J. Manuf. Sci. Eng. 143, 041013 (2021).
Cook, R. F. & Oyen, M. L. On the failure and fracture of hydrogels for cartilage replacement. J. Phys. Mater. 4, 021001 (2021).
Ilseng, A., Skallerud, H., Stokke, B. T. & Prot, V. A perturbation analysis approach for studying the effect of swelling kinetics on instabilities in hydrogel plates. J. Appl. Mech. 88, 051002 (2021).
Hu, C., Long, L., Cao, J., Zhang, S. & Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 411, 128564 (2021).
Das, S. & Roy, D. A poroviscoelasticity model based on effective temperature for water and temperature driven phase transition in hydrogels. Int. J. Mech. Sci. 196, 106290 (2021).
Yuan, S. et al. Dual 3-D networked Pickering emulsion hydrogels encapsulating copper extractants for the recovery of Cu2+ from water. J. Environ. Chem. Eng. 9, 105154 (2021).
Nakhjiri, M. T., Bagheri Marandi, G. & Kurdtabar, M. Preparation of magnetic double network nanocomposite hydrogel for adsorption of phenol and p-nitrophenol from aqueous solution. J. Environ. Chem. Eng. 9, 105039 (2021).
Zhu, D. et al. Single injection and multiple treatments: An injectable nanozyme hydrogel as AIEgen reservoir and release controller for efficient tumor therapy. Nano Today 37, 101091 (2021).
Garg, D., Matai, I. & Sachdev, A. Toward designing of anti-infective hydrogels for orthopedic implants: from lab to clinic. ACS Biomater. Sci. Eng. 7, 1933 (2021).
Park, D., Kim, J. W., Shin, K. & Kim, J. W. Bacterial cellulose nanofibrils-reinforced composite hydrogels for mechanical compression-responsive on-demand drug release. Carbohydr. Polym. 272, 118459 (2021).
Macdougall, L. J. et al. Self-healing, stretchable and robust interpenetrating network hydrogels. Biomater. Sci. 6, 2932 (2018).
Zhao, L. et al. Fast water transport reversible CNT/PVA hybrid hydrogels with highly environmental tolerance for multifunctional sport headband. Compos. Part B Eng. 211, 108661 (2021).
Xin, T. et al. Inorganic strengthened hydrogel membrane as regenerative periosteum. ACS Appl. Mater. Interfaces 9, 41168 (2017).
Xin, T. et al. Programmed sustained release of recombinant human bone morphogenetic protein-2 and inorganic ion composite hydrogel as artificial periosteum. ACS Appl. Mater. Interfaces 12, 6840 (2020).
Abalymov, A. et al. Alkaline phosphatase delivery system based on calcium carbonate carriers for acceleration of ossification. ACS Appl. Bio Mater. 3, 2986 (2021).
Huang, K., Wu, J. & Gu, Z. Black phosphorus hydrogel scaffolds enhance bone regeneration via a sustained supply of calcium-free phosphorus. ACS Appl. Mater. Interfaces 11, 2908 (2018).
Radhakrishnan, J., Manigandan, A., Chinnaswamy, P., Subramanian, A. & Sethuraman, S. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials 162, 82 (2018).
Shen, J., Dai, Y., Xia, F. & Zhang, X. Role of divalent metal ions in the function and application of hydrogels. Prog. Polym. Sci. 135, 101622 (2022).
Zhu, T. et al. Recent advances in conductive hydrogels: classifications, properties, and applications. Chem. Soc. Rev. 52, 473–509 (2023).
Li, W. et al. Nanoconfined polymerization limits crack propagation in hysteresis-free gels. Nat. Mater. 23, 131 (2023).
Qu, G. et al. Emission enhancement and self-healing of a hybrid hydrogel employing Au nanoclusters as cross-linkers. Dyes Pigments 188, 109211 (2021).
Duc, T. H. et al. Synthesis and application of hydrogel calcium alginate microparticles as a biomaterial to remove heavy metals from aqueous media. Environ. Technol. Innov. 22, 101400 (2021).
Mallakpour, S., Behranvand, V. & Mallakpour, F. Decolourization of textile dyes using CNT-based hybrid materials. J. Environ. Chem. Eng. 9, 105170 (2021).
He, P. et al. Tough and super-stretchable conductive double network hydrogels with multiple sensations and moisture-electric generation. Chem. Eng. J. 414, 128726 (2021).
Yan, L. et al. Surfactin-reinforced gelatin methacrylate hydrogel accelerates diabetic wound healing by regulating the macrophage polarization and promoting angiogenesis. Chem. Eng. J. 414, 128836 (2021).
Zhang, X., Zhang, Y., Zhang, W., Dai, Y. & Xia, F. Gold nanoparticles-deranged double network for Janus adhesive-tough hydrogel as strain sensor. Chem. Eng. J. 420, 130447 (2021).
Zhou, L., Chen, F., Hou, Z., Chen, Y. & Luo, X. Injectable self-healing CuS nanoparticle complex hydrogels with antibacterial, anti-cancer, and wound healing properties. Chem. Eng. J. 409, 128224 (2021).
Fakir, A. et al. Engineering of new hydrogel beads based conducting polymers: Metal-free catalysis for highly organic pollutants degradation. Appl. Catal. B Environ. 286, 119948 (2021).
Zhang, X. et al. Role of a high calcium ion content in extending the properties of alginate dual-crosslinked hydrogels. J. Mater. Chem. A 8, 25390–25401 (2020).
Zhang, W., Zhang, Y., Dai, Y. & Xia, F. Gradient adhesion modification of polyacrylamide/alginate–calcium tough hydrogels. J. Mater. Chem. B 10, 757–764 (2022).
Bayanati, M. et al. Nanosilver/hydrogel: Synthesis and application in delaying senescence of cut flower. S. Afr. J. Bot. 138, 415 (2021).
Wang, R. et al. Facile preparation of agar/polyvinyl alcohol-based triple-network composite hydrogels with excellent mechanical performances. Colloid. Surface. A 615, 126270 (2021).
Wu, K. et al. Reinforced polyaniline-dodecyl benzene sulfonate hydrogel with well-aligned fibrous morphology as durable electrode materials for Zn-ion battery. Synthetic Metals 274, 116721 (2021).
Jiang, T., Zhao, X., Yin, X., Yang, R. & Tan, G. Dynamically adaptive window design with thermo-responsive hydrogel for energy efficiency. Appl. Energy 287, 116573 (2021).
Hasani-Sadrabadi, M. M. et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 12, eaay6853 (2020).
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (Nos. 81772433 and 62304148), the Great Discipline Construction Project from Minhang District, Shanghai (No. 2017MWDXK04), Research in Cutting-Edge Technology of Suzhou (No. SYG202351), and the China Postdoctoral Science Foundation (No. 2023M730653).
Author information
Authors and Affiliations
Contributions
W. Ding and Y.X. Ge contributed equally to this work. All the authors contributed to the writing, review, and editing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ding, W., Ge, Y., Zhang, T. et al. Advanced construction strategies to obtain nanocomposite hydrogels for bone repair and regeneration. NPG Asia Mater 16, 14 (2024). https://doi.org/10.1038/s41427-024-00533-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-024-00533-z