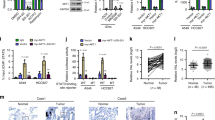
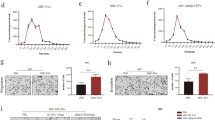
Abstract
The high mortality rate associated with melanoma primarily results from metastasis and recurrence. However, the precise mechanisms driving these processes remain poorly understood. Intercellular communication between cancer cells and non-cancer cells significantly influences the tumor microenvironment and plays a crucial role in metastasis. Therefore, our current study aims to investigate the role and mechanism of long non-coding RNAs (lncRNAs) in regulating the interaction between melanoma cancer stem cells (CSCs) and non-CSCs during the metastatic colonization process. This study has characterized a novel lncRNA called Gm33149. Importantly, we provide evidence for the first time that Gm33149, originating from highly metastatic melanoma stem cells (OL-SD), can be packaged into exosomes and transferred to low-metastatic nonstem cells (OL). Once internalized by OL cells, Gm33149 exerts its function through a competitive endogenous RNA mechanism (ceRNA) involving miR-5623-3p. Specifically, Gm33149 competitively binds to miR-5623-3p, thereby activating the Wnt signaling pathway and promoting the acquisition of a more aggressive metastatic phenotype by OL cells. In summary, our findings suggest that targeting lncRNA Gm33149 within extracellular vesicles could potentially serve as a therapeutic strategy for the treatment of metastatic melanoma.

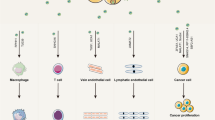
Schematic representation of the mechanisms underlying the pro-metastatic activity of lncRNA Gm33149 mediated by exosomal transfer. The figure illustrates the key mechanisms involved in the pro-metastatic activity of lncRNA Gm33149 through exosomal transfer. Melanoma stem cells (OLSD) release exosomes containing lncRNA Gm33149. These exosomes are taken up by non-stem melanoma cells (OL), delivering lncRNA Gm33149 to the recipient cells. Within OL cells, lncRNA Gm33149 functions as a competitive endogenous RNA (ceRNA), sequestering miR-5623-3p. This sequestration prevents miR-5623-3p from binding to its target genes, thereby activating the Wnt signaling pathway. The activated Wnt signaling pathway enhances the migration, invasion, and metastatic colonization capabilities of OL cells. The transfer of lncRNA Gm33149 via exosomes contributes to OL cells acquiring “metastatic competency” while promoting their metastatic colonization. These findings underscore the importance of lncRNA Gm33149 in intercellular communication and the metastatic progression of melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Ahmed B, Qadir MI, Ghafoor S. Malignant Melanoma: Skin Cancer-Diagnosis, Prevention, and Treatment. Crit Rev Eukaryot Gene Expr. 2020;30:291–7.
Kalaora S, Nagler A, Wargo JA, Samuels Y. Mechanisms of immune activation and regulation: lessons from melanoma. Nat Rev Cancer. 2022;22:195–207.
Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J (Sudbury, Mass). 2014;20:256–61.
Swoboda A, Soukup R, Eckel O, Kinslechner K, Wingelhofer B, Schörghofer D, et al. STAT3 promotes melanoma metastasis by CEBP-induced repression of the MITF pathway. Oncogene. 2021;40:1091–105.
Botti G, Fratangelo F, Cerrone M, Liguori G, Cantile M, Anniciello AM, et al. COX-2 expression positively correlates with PD-L1 expression in human melanoma cells. J Transl Med. 2017;15:46.
Marzagalli M, Ebelt ND, Manuel ER. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Cancer Biol. 2019;59:236–50.
Peña-Romero AC, Orenes-Piñero E. Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers. Cancers. 2022;14:1681.
Patton EE, Mueller KL, Adams DJ, Anandasabapathy N, Aplin AE, Bertolotto C, et al. Melanoma models for the next generation of therapies. Cancer cell. 2021;39:610–31.
Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019;177:414–27.e413.
García-Silva S, Benito-Martín A, Nogués L, Hernández-Barranco A, Mazariegos MS, Santos V, Hergueta-Redondo M, Ximénez-Embún P, et al. Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nat cancer. 2021;2:1387–405.
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8.
Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234:8381–95.
Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25:20.
Munro MJ, Wickremesekera SK, Peng L, Tan ST, Itinteang T. Cancer stem cells in colorectal cancer: a review. J Clin Pathol. 2018;71:110–6.
Muraoka D, Seo N, Hayashi T, Tahara Y, Fujii K, Tawara I, et al. Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance, The. J Clin Investig. 2019;129:1278–94.
Eun K, Ham SW, Kim H. Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep. 2017;50:117–25.
Liu H, Luo J, Luan S, He C, Li Z. Long non-coding RNAs involved in cancer metabolic reprogramming. Cell Mol Life Sci: CMLS. 2019;76:495–504.
Li J, Meng H, Bai Y, Wang K. Regulation of lncRNA and Its Role in Cancer Metastasis. Oncol Res. 2016;23:205–17.
ZHANG ZX. Shi-ping, Research progress on role of Wnt signaling pathway in regulation of tumors[J]. Chin Pharmacol Bull. 2017;33(1):14–17,18.
Gambi G, Mengus G, Davidson G, Demesmaeker E, Cuomo A, Bonaldi T, et al. The lncRNA LENOX Interacts with RAP2C to Regulate Metabolism and Promote Resistance to MAPK Inhibition in Melanoma. Cancer Res. 2022;82:4555–70.
Li Y, Gao Y, Niu X, Tang M, Li J, Song B, et al. lncRNA BASP1-AS1 interacts with YBX1 to regulate Notch transcription and drives the malignancy of melanoma. Cancer Sci. 2021;112:4526–42.
Liu Y, Zhuang Y, Fu X, Li C. lncRNA POU3F3 promotes melanoma cell proliferation by downregulating lncRNA MEG3, Discover. Oncology. 2021;12:21.
Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol/Hematol. 2016;99:141–9.
Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–73.
Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165.
de Sousa EMF, Vermeulen L. Wnt Signaling in Cancer Stem Cell Biology. Cancers. 2016;8:60.
Zhang Y, Chen Y, Shi L, Li J, Wan W, Li B, et al. Extracellular vesicles microRNA-592 of melanoma stem cells promotes metastasis through activation of MAPK/ERK signaling pathway by targeting PTPN7 in non-stemness melanoma cells. Cell Death Discov. 2022;8:428.
Liu D, Li X, Zeng B, Zhao Q, Chen H, Zhang Y, et al. H.R. Xing, Exosomal microRNA-4535 of Melanoma Stem Cells Promotes Metastasis by Inhibiting Autophagy Pathway. Stem cell Rev Rep. 2023;19:155–69.
Li X, Liu D, Chen H, Zeng B, Zhao Q, Zhang Y, et al. H.R. Xing, Melanoma stem cells promote metastasis via exosomal miR-1268a inactivation of autophagy. Biol Res. 2022;55:29.
Weiss F, Lauffenburger D, Friedl P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat Rev Cancer. 2022;22:157–73.
Wang X, Huang J, Chen W, Li G, Li Z, Lei J. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp Mol Med. 2022;54:1390–400.
Marte B. Tumour heterogeneity. Nature. 2013;501:327.
Zhao S, Guan B, Mi Y, Shi D, Wei P, Gu Y, et al. lncRNA MIR17HG promotes colorectal cancer liver metastasis by mediating a glycolysis-associated positive feedback circuit. Oncogene. 2021;40:4709–24.
Möller A, Lobb RJ. The evolving translational potential of small extracellular vesicles in cancer. Nat Rev Cancer. 2020;20:697–709.
Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, Tu Q, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat cell Biol. 2019;21:498–510.
Zhang W, Wang Q, Yang Y, Zhou S, Zhang P, Feng T. The role of exosomal lncRNAs in cancer biology and clinical management. Exp Mol Med. 2021;53:1669–73.
Yu Z, Tang H, Chen S, Xie Y, Shi L, Xia S, et al. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resistance Updates Rev Commentaries Antimicrobial Anticancer Chemother. 2023;67:100915.
Chen H, Zeng B, Li X, Zhao Q, Liu D, Chen Y, et al. High-Metastatic Melanoma Cells Promote the Metastatic Capability of Low-Metastatic Melanoma Cells via Exosomal Transfer of miR-411-5p. Front Oncol. 2022;12:895164.
Wang CG, Hu YH, Su SL, Zhong D. LncRNA DANCR and miR-320a suppressed osteogenic differentiation in osteoporosis by directly inhibiting the Wnt/β-catenin signaling pathway. Exp Mol Med. 2020;52:1310–25.
Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan C, Xu M, Sun H, Liu C, Wei P, Du X. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J Hematol Oncol. 2018;11:113.
Jang SC, Economides KD, Moniz RJ, Sia CL, Lewis N, McCoy C, et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance, Commun. Biol. 2021;4:497.
Zhang Z, Zhou C, Chang Y, Zhang Z, Hu Y, Zhang F, et al. Long non-coding RNA CASC11 interacts with hnRNP-K and activates the WNT/β-catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett. 2016;376:62–73.
Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, et al. LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer. 2018;17:126.
Acknowledgements
Throughout the writing of this dissertation, I have received a great deal of support and assistance. I would first like to thank my supervisor, H. Rosie Xing, whose expertise was invaluable in formulating the research questions and methodology. I would also like to thank my tutor, Jian-Yu Wang, for their valuable guidance throughout my studies. I would particularly like to acknowledge my team members, Yu-Han Zhang, Jie Li, Lei Shi, Jia-Cheng Xie, Xue Han, Yuting Chen, Meng Xiang, and Bo-Wen Li, for their wonderful collaboration and patient support.
Funding
This work was supported by the National Natural Science Fund (Grant No. 82073277 and 82173247), the Science and Technology Project Affiliated to the Education Department of Chongqing (Grant No. KJQN202100404), and Project of Chongqing Natural Science Foundation Innovation and Development Fund (Municipal Education Commission) (Grant No. CSTB2022NS CQ-LZX0023).
Author information
Authors and Affiliations
Contributions
YC, YH Z and JL conducted the experiments and analyzed the data. YC was responsible for writing the manuscript. LS, JC X, XH, YT C, MX and BW L participated in the conduction of this study. JY W and HR X designed and oversaw the execution of this study, and contributed to the writing of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The Author confirms: that the work described has not been published before; that it is not under consideration for publication elsewhere; and that its publication has been approved by all authors.
Ethical approval
Animal experiments were approved by the Chongqing Medical University committee for animal experiments. All experiments were performed with relevant guidelines and regulations. Title: Novel lncRNA Gm33149 Modulates Metastatic Heterogeneity in Melanoma by Regulating the miR-5623-3p/Wnt Axis via Exosomal Transfer; the institutional approval committee: Chongqing Medical University committee; approval number: SYXK2018-0003; date: 2022-02-22\2022-06-23.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Zhang, YH., Li, J. et al. Novel lncRNA Gm33149 modulates metastatic heterogeneity in melanoma by regulating the miR-5623-3p/Wnt axis via exosomal transfer. Cancer Gene Ther 31, 364–375 (2024). https://doi.org/10.1038/s41417-023-00707-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-023-00707-x
This article is cited by
-
Alternative Wnt-signaling axis leads to a break of oncogene-induced senescence
Cell Death & Disease (2024)