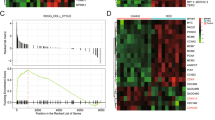
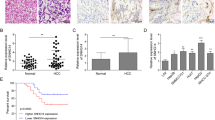
Abstract
Increasing evidence has suggested the crucial role cyclin-dependent kinases (CDKs) in the biology of hepatocellular carcinoma (HCC), a lethal malignancy with high morbidity and mortality. Hence, this study explored the modulatory effect of the putative cyclin-dependent kinase 11B (CDK11B)-mediated ubiquitination on HCC stem cells. The expression of CDK11B, SAM pointed domain-containing ETS transcription factor (SPDEF) and DOT1-like histone lysine methyltransferase (DOT1L) was determined by RT-qPCR and western blot analysis in HCC tissues and cells. The interaction among CDK11B, SPDEF, miR-448, and DOT1L was analyzed by Co-IP, ubiquitination-IP and ChIP assays, whereas their effects on the biological characteristics of HCC stem cells were assessed by sphere formation and colony formation assays. An in vivo xenograft tumor model was developed for validating the regulation of CDK11B in oncogenicity of HCC stem cells. We characterized the aberrant upregulation of CDK11B and downregulation SPDEF in HCC tissues and cells. CDK11B degraded SPDEF through ubiquitin-proteasome pathway, whereas SPDEF could bind to the miR-448 promoter and inhibit the expression of DOT1L by activating miR-448, whereby promoting self-renewal of HCC stem cells. Knockdown of CDK11B attenuated the self-renewal capability of HCC stem cells and their oncogenicity in vivo. These findings highlighted that blocking the CDK11B-induced degradation of SPDEF and enhancing miR-448-dependent inhibition of DOT1L may delay the progression of HCC by restraining self-renewal capability of HCC stem cells, representing novel targets for HCC management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shimada S, Mogushi K, Akiyama Y, Furuyama T, Watanabe S, Ogura T, et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 2019;40:457–70.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–14.
Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019;156:477–91. e1.
Santopaolo F, Lenci I, Milana M, Manzia TM, Baiocchi L. Liver transplantation for hepatocellular carcinoma: where do we stand? World J Gastroenterol. 2019;25:2591–602.
Liao Y, Feng Y, Shen J, Hornicek FJ, Duan Z. The roles and therapeutic potential of cyclin-dependent kinases (CDKs) in sarcoma. Cancer Metastasis Rev. 2016;35:151–63.
Shen S, Dean DC, Yu Z, Duan Z. Role of cyclin-dependent kinases (CDKs) in hepatocellular carcinoma: therapeutic potential of targeting the CDK signaling pathway. Hepatol Res. 2019;49:1097–108.
Alonso MH, Ausso S, Lopez-Doriga A, Cordero D, Guino E, Sole X, et al. Comprehensive analysis of copy number aberrations in microsatellite stable colon cancer in view of stromal component. Br J Cancer. 2017;117:421–31.
Tamura RE, Paccez JD, Duncan KC, Morale MG, Simabuco FM, Dillon S, et al. GADD45alpha and gamma interaction with CDK11p58 regulates SPDEF protein stability and SPDEF-mediated effects on cancer cell migration. Oncotarget 2016;7:13865–79.
Meiners J, Schulz K, Moller K, Hoflmayer D, Burdelski C, Hube-Magg C, et al. Upregulation of SPDEF is associated with poor prognosis in prostate cancer. Oncol Lett. 2019;18:5107–18.
Wu J, Qin W, Wang Y, Sadik A, Liu J, Wang Y, et al. SPDEF is overexpressed in gastric cancer and triggers cell proliferation by forming a positive regulation loop with FoxM1. J Cell Biochem. 2018;119:9042–54.
Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314.
Zhu H, Zhou X, Ma C, Chang H, Li H, Liu F, et al. Low expression of miR-448 induces EMT and promotes invasion by regulating ROCK2 in hepatocellular carcinoma. Cell Physiol Biochem. 2015;36:487–98.
Guo JC, Yang YJ, Zhang JQ, Guo M, Xiang L, Yu SF, et al. microRNA-448 inhibits stemness maintenance and self-renewal of hepatocellular carcinoma stem cells through the MAGEA6-mediated AMPK signaling pathway. J Cell Physiol. 2019;234:23461–74.
Gibbons GS, Chakraborty A, Grigsby SM, Umeano AC, Liao C, Moukha-Chafiq O, et al. Identification of DOT1L inhibitors by structure-based virtual screening adapted from a nucleoside-focused library. Eur J Med Chem. 2020;189:112023.
Lv L, Li Q, Chen S, Zhang X, Tao X, Tang X, et al. miR-133b suppresses colorectal cancer cell stemness and chemoresistance by targeting methyltransferase DOT1L. Exp Cell Res. 2019;385:111597.
Reish NJ, Maltare A, McKeown AS, Laszczyk AM, Kraft TW, Gross AK, et al. The age-regulating protein klotho is vital to sustain retinal function. Invest Ophthalmol Vis Sci. 2013;54:6675–85.
Chen EB, Zhou SL, Pang XG, Yin D, Miao PZ, Yang Y, et al. Prostate-derived ETS factor improves prognosis and represses proliferation and invasion in hepatocellular carcinoma. Oncotarget 2017;8:52488–500.
Shao YY, Li YS, Hsu HW, Lin H, Wang HY, Wo RR, et al. Potent activity of composite cyclin dependent kinase inhibition against hepatocellular carcinoma. Cancers (Basel). 2019;11:1433.
Haider C, Grubinger M, Reznickova E, Weiss TS, Rotheneder H, Miklos W, et al. Novel inhibitors of cyclin-dependent kinases combat hepatocellular carcinoma without inducing chemoresistance. Mol Cancer Ther. 2013;12:1947–57.
Ehrlich SM, Liebl J, Ardelt MA, Lehr T, De Toni EN, Mayr D, et al. Targeting cyclin dependent kinase 5 in hepatocellular carcinoma–a novel therapeutic approach. J Hepatol. 2015;63:102–13.
Yin T, Liu MM, Jin RT, Kong J, Wang SH, Sun WB. miR-152-3p Modulates hepatic carcinogenesis by targeting cyclin-dependent kinase 8. Pathol Res Pract. 2019;215:152406.
Liu TH, Wu YF, Dong XL, Pan CX, Du GY, Yang JG, et al. Identification and characterization of the BmCyclin L1-BmCDK11A/B complex in relation to cell cycle regulation. Cell Cycle. 2017;16:861–8.
Xiao B, Kuang Z, Zhang W, Hang J, Chen L, Lei T, et al. Glutamate ionotropic receptor kainate type subunit 3 (GRIK3) promotes epithelial-mesenchymal transition in breast cancer cells by regulating SPDEF/CDH1 signaling. Mol Carcinog. 2019;58:1314–23.
Lo YH, Noah TK, Chen MS, Zou W, Borras E, Vilar E, et al. SPDEF induces quiescence of colorectal cancer cells by changing the transcriptional targets of beta-catenin. Gastroenterology 2017;153:205–18. e8.
Motti ML, S DA, Meccariello R. MicroRNAs, cancer and diet: facts and new exciting perspectives. Curr Mol Pharm. 2018;11:90–6.
Qi H, Wang H, Pang D. miR-448 promotes progression of non-small-cell lung cancer via targeting SIRT1. Exp Ther Med. 2019;18:1907–13.
Lou Q, Liu R, Yang X, Li W, Huang L, Wei L, et al. miR-448 targets IDO1 and regulates CD8(+) T cell response in human colon cancer. J Immunother Cancer. 2019;7:210.
Ivey KN, Srivastava D. microRNAs as developmental regulators. Cold Spring Harb Perspect Biol. 2015;7:a008144.
Yang L, Lei Q, Li L, Yang J, Dong Z, Cui H. Silencing or inhibition of H3K79 methyltransferase DOT1L induces cell cycle arrest by epigenetically modulating c-Myc expression in colorectal cancer. Clin Epigenetics. 2019;11:199.
Sarno F, Nebbioso A, Altucci L. DOT1L: a key target in normal chromatin remodelling and in mixed-lineage leukaemia treatment. Epigenetics. 2019;15:439–53.
Bourguignon LY, Wong G, Shiina M. Up-regulation of histone methyltransferase, DOT1L, by matrix hyaluronan promotes MicroRNA-10 expression leading to tumor cell invasion and chemoresistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2016;291:10571–85.
Acknowledgements
We acknowledge and appreciate our colleagues for their valuable suggestions and technical assistance for this study. This work was supported by the Key Research and Development Plan of Hainan Province (2019RC373) and Hainan Natural Science Foundation (818MS161).
Author information
Authors and Affiliations
Contributions
Jun-cheng Guo and Yi-jun Yang designed the study. Min Guo, Jian-quan Zhang, and Jin-fang Zheng collated the data, carried out data analyses and produced the initial draft of the manuscript. Zhuo Liu contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Guo, Jc., Yang, Yj., Guo, M. et al. Involvement of CDK11B-mediated SPDEF ubiquitination and SPDEF-mediated microRNA-448 activation in the oncogenicity and self-renewal of hepatocellular carcinoma stem cells. Cancer Gene Ther 28, 1136–1149 (2021). https://doi.org/10.1038/s41417-020-00261-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00261-w