Abstract
Background
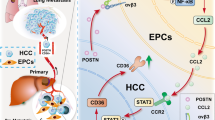
This study aimed to investigate the underlying mechanisms of matricellular protein periostin (POSTN) on tumour-stroma crosstalk in the liver metastatic microenvironment of colorectal cancer (CRC).
Methods
Postn-knockout mice and hepatic Postn-overexpressing mice were used to investigate the functions of POSTN on the formation of fibrotic microenvironment and the tumour-stroma crosstalk in the liver metastatic microenvironment of CRC. Clinical samples and database were analyzed to show the correlation between POSTN expression and fibrotic features and TGF-β signalling in metastatic livers of CRC.
Results
POSTN deficiency reduced hepatic stellate cell (HSC) activation and liver metastasis, whereas POSTN overexpression in the liver significantly augmented the formation of a fibrotic microenvironment to support the liver metastatic growth of CRC cells in mice. Moreover, HSC-derived POSTN promoted TGF-β1 expression in CRC cells through the integrin/FAK/ERK/STAT3 pathway; conversely, tumour cell-derived TGF-β1 induced POSTN expression in HSCs via the Smad pathway. POSTN levels correlated with fibrotic features and TGF-β signalling in metastatic liver tissues of CRC patients.
Conclusions
POSTN and TGF-β1 cooperatively contribute to the tumour-stroma crosstalk by forming a supporting fibrotic microenvironment to promote liver metastasis of CRC cells via the POSTN/integrin/FAK/ERK/STAT3/TGF-β axis in tumour cells and TGF-β/Smad/POSTN signalling in activated HSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are present in the manuscript and the Supplementary Materials. Additional data related to this paper may be requested from the corresponding author.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D, et al. Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov. 2022;12:134–53.
Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021;39:708–24.e11.
Zhou H, Liu Z, Wang Y, Wen X, Amador EH, Yuan L, et al. Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther. 2022;7:70.
Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–84.
Ouahoud S, Voorneveld PW, van der Burg LRA, de Jonge-Muller ESM, Schoonderwoerd MJA, Paauwe M, et al. Bidirectional tumor/stroma crosstalk promotes metastasis in mesenchymal colorectal cancer. Oncogene. 2020;39:2453–66.
Zhao S, Mi Y, Zheng B, Wei P, Gu Y, Zhang Z, et al. Highly-metastatic colorectal cancer cell released miR-181a-5p-rich extracellular vesicles promote liver metastasis by activating hepatic stellate cells and remodelling the tumour microenvironment. J Extracell Vesicles. 2022;11:e12186.
Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54.
Kai F, Drain AP, Weaver VM. The extracellular matrix modulates the metastatic journey. Dev Cell. 2019;49:332–46.
Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303.
Huang Y, Liu W, Xiao H, Maitikabili A, Lin Q, Wu T, et al. Matricellular protein periostin contributes to hepatic inflammation and fibrosis. Am J Pathol. 2015;185:786–97.
Ono J, Takai M, Kamei A, Azuma Y, Izuhara K. Pathological roles and clinical usefulness of periostin in type 2 inflammation and pulmonary fibrosis. Biomolecules. 2021;11:1084.
Nanri Y, Nunomura S, Honda Y, Takedomi H, Yamaguchi Y, Izuhara K A positive loop formed by SOX11 and periostin upregulates TGF-β signals leading to skin fibrosis. J Invest Dermatol. (2022):S0022-202X(22)02902–5.
Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–9.
Wang Z, Ouyang G. Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell. 2012;10:111–2.
Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014;71:1279–88.
Ma Z, Zhao X, Deng M, Huang Z, Wang J, Wu Y, et al. Bone marrow mesenchymal stromal cell-derived periostin promotes B-ALL progression by modulating CCL2 in leukemia cells. Cell Rep. 2019;26:1533–43.e4.
Ma H, Wang J, Zhao X, Wu T, Huang Z, Chen D, et al. Periostin promotes colorectal tumorigenesis through integrin-FAK-Src pathway-mediated YAP/TAZ activation. Cell Rep. 2020;30:793–806.e6.
Wei T, Wang K, Liu S, Fang Y, Hong Z, Liu Y, et al. Periostin deficiency reduces PD-1+ tumor-associated macrophage infiltration and enhances anti-PD-1 efficacy in colorectal cancer. Cell Rep. 2023;42:112090.
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–39.
Lu Y, Liu X, Jiao Y, Xiong X, Wang E, Wang X, et al. Periostin promotes liver steatosis and hypertriglyceridemia through downregulation of PPARα. J Clin Invest. 2014;124:3501–13.
Xiao H, Zhang Y, Li Z, Liu B, Cui D, Liu F, et al. Periostin deficiency reduces diethylnitrosamine-induced liver cancer in mice by decreasing hepatic stellate cell activation and cancer cell proliferation. J Pathol. 2021;255:212–23.
Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, et al. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258–62.
Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-β pathway. Proc Natl Acad Sci USA. 2012;109:10978–83.
Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-β1. J Exp Med. 2006;203:1021–31.
Chen G, Nakamura I, Dhanasekaran R, Iguchi E, Tolosa EJ, Romecin PA, et al. Transcriptional induction of periostin by a sulfatase 2-TGFβ1-SMAD signaling axis mediates tumor angiogenesis in hepatocellular carcinoma. Cancer Res. 2017;77:632–45.
Tan HX, Gong WZ, Zhou K, Xiao ZG, Hou FT, Huang T, et al. CXCR4/TGF-β1 mediated hepatic stellate cells differentiation into carcinoma-associated fibroblasts and promoted liver metastasis of colon cancer. Cancer Biol Ther. 2020;21:258–68.
Badiola I, Olaso E, Crende O, Friedman SL, Vidal-Vanaclocha F. Discoidin domain receptor 2 deficiency predisposes hepatic tissue to colon carcinoma metastasis. Gut. 2012;61:1465–72.
Reszegi A, Horváth Z, Karászi K, Regős E, Postniková V, Tátrai P, et al. The protective role of decorin in hepatic metastasis of colorectal carcinoma. Biomolecules. 2020;10:1199.
Coulouarn C. Modulating the activation of hepatic stellate cells: a cunning way for metastatic cells to create a permissive soil for seeding in the liver? Hepatology. 2015;61:37–40.
Tsilimigras DI, Brodt P, Clavien PA, Muschel RJ, D’Angelica MI, Endo I, et al. Liver metastases. Nat Rev Dis Primers. 2021;7:27.
Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–64.
Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–60.
Wang Y, Tu K, Liu D, Guo L, Chen Y, Li Q, et al. p300 acetyltransferase is a cytoplasm-to-nucleus shuttle for SMAD2/3 and TAZ nuclear transport in transforming growth factor β-stimulated hepatic stellate cells. Hepatology. 2019;70:1409–23.
Qi M, Fan S, Huang M, Pan J, Li Y, Miao Q, et al. Targeting FAPα-expressing hepatic stellate cells overcomes resistance to antiangiogenics in colorectal cancer liver metastasis models. J Clin Invest. 2022;132:e157399.
Acknowledgements
We are deeply grateful to Prof. Xiaoying Li for providing recombinant adenoviruses and Dr. Fan Liu and Dr. Dan Cui for their valuable support.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81772616, 81470793, 81972748, 82172932, and 82273416) and the Natural Science Foundation of Fujian Province of China (2019J02002).
Author information
Authors and Affiliations
Contributions
BL, TW, BLin, XL and CF acquired experimental data. BL, TW, BLin, GS, CF and GO were involved in manuscript writing. BL, TW, BLin, YL, GS, CF and GO analyzed the data. TW, GS and GO obtained funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Human clinical samples were collected with informed consent from the patients. The collection and study of human clinical samples were performed in accordance with the approved guidelines of the Ethics and Scientific Committees of Xiamen Hospital of Traditional Chinese Medicine.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, B., Wu, T., Lin, B. et al. Periostin–TGF-β feedforward loop contributes to tumour-stroma crosstalk in liver metastatic outgrowth of colorectal cancer. Br J Cancer 130, 358–368 (2024). https://doi.org/10.1038/s41416-023-02516-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02516-3