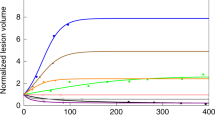
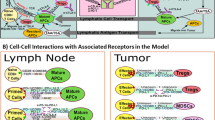
Abstract
Longitudinal models of biomarkers such as tumour size dynamics capture treatment efficacy and predict treatment outcome (overall survival) of a variety of anticancer therapies, including chemotherapies, targeted therapies, immunotherapies and their combinations. These pharmacological endpoints like tumour dynamic (tumour growth inhibition) metrics have been proposed as alternative endpoints to complement the classical RECIST endpoints (objective response rate, progression-free survival) to support early decisions both at the study level in drug development as well as at the patients level in personalised therapy with checkpoint inhibitors. This perspective paper presents recent developments and future directions to enable wider and robust use of model-based decision frameworks based on pharmacological endpoints.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. EuropeanOrganization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Food and Drug Administration Oncologic Drugs Advisory Committee, April 27-29, 2021. https://www.fda.gov/advisory-committees/advisory-committee-calendar/april-27-29-2021-meeting-oncologic-drugs-advisory-committee-meeting-announcement-04272021-04292021#event-materials. Accessed October 27, 2022.
Michaelis LC, Ratain MJ. Measuring response in a post-RECIST world: from black and white to shades of grey. Nat Rev Cancer. 2006;6:409–14.
Claret L, Girard P, O’Shaughnessy J, Hoff P, Van Cutsem E, Blum J, et al. Model-based predictions of expected anti-tumor response and survival in phase III studies based on phase II data of an investigational agent. J Clin Oncol. 2006;24:6025–6025.
Gong Y, Mason J, Shen YL, Chang E, Kazandjian D, Blumenthal GM, et al. An FDA analysis of the association of tumor growth rate and overall and progression-free survival in metastatic non-small cell lung cancer (NSCLC) patients. J Clin Oncol. 2020;38:9541–9541.
Maitland ML, Wilkerson J, Karovic S, Zhao B, Flynn J, Zhou M, et al. Enhanced detection of treatment effects on metastatic colorectal cancer with volumetric CT measurements for tumor burden growth rate evaluation. Clin Cancer Res. 2020;26:6464–74.
Bruno R, Marchand M, Yoshida K, Chan P, Li H, Zhu W, et al. Tumor dynamic model-based decision support for Phase Ib/II combination studies: a retrospective assessment based on resampling of the Phase III study IMpower150. Clin Cancer Res. 2023; https://doi.org/10.1158/1078-0432.CCR-22-2323.
Kerioui M, Desmée S, Mercier F, Lin A, Wu B, Jin JY, et al. Assessing the impact of organ-specific lesion dynamics on survival in patients with recurrent urothelial carcinoma treated with atezolizumab or chemotherapy. ESMO Open. 2022;7:100346.
Kerioui M, Desmée S, Bertrand J, Le Tourneau C, Mercier F, Bruno R, et al. Assessing the increased variability in individual lesion kinetics during immunotherapy: does it exist, and does it matter? J Clin Oncol Precision Oncol. in press.
Wilkerson J, Abdallah K, Hugh-Jones C, Curt G, Rothenberg M, Simantov R, et al. Estimation of tumour regression and growth rates during treatment in patients with advanced prostate cancer: a retrospective analysis. Lancet Oncol. 2017;18:143–54.
Colomban O, Tod M, Leary A, Ray-Coquard I, Lortholary A, Hardy-Bessard AC, et al. Early modeled longitudinal CA-125 kinetics and survival of ovarian cancer patients: a GINECO AGO MRC CTU study. Clin Cancer Res. 2019;25:5342–50.
Jonsson F, Ou Y, Claret L, Siegel D, Jagannath S, Vij R, et al. A tumor growth inhibition model based on M-protein levels in subjects with relapsed/refractory multiple myeloma following single-agent carfilzomib use. CPT Pharmacomet Syst Pharm. 2015;4:711–9.
Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022;21:79.
Bratman SV, Yang SYC, Lafolla MAJ, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1:873–81.
Weber S, van der Leest P, Donker HC, Schlange T, Timens W, Tamminga M, et al. Dynamic changes of circulating tumor DNA predict clinical outcome in patients with advanced non-small-cell lung cancer treated with immune checkpoint inhibitors. JCO Precis Oncol. 2021;5:1540–53.
Zou W, Yaung SJ, Fuhlbrück F, Ballinger M, Peters E, Palma JF, et al. ctDNA predicts overall survival in patients with NSCLC treated with PD-L1 blockade or with chemotherapy. JCO Precis Oncol. 2021;5:827–38.
Food and Drug Administration. Use of Circulating Tumor DNA for Early-Stage Solid Tumor Drug Development - Guidance for Industry 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-circulating-tumor-deoxyribonucleic-acid-early-stage-solid-tumor-drug-development-draft-guidance. Accessed February 6, 2023.
Yin A, van Hasselt JGC, Guchelaar HJ, Friberg LE, Moes DJAR. Anti-cancer treatment schedule optimization based on tumor dynamics modelling incorporating evolving resistance. Sci Rep. 2022;12:4206.
Janssen JM, Verheijen RB, van Duijl TT, Lin L, van den Heuvel MM, Beijnen JH, et al. Longitudinal nonlinear mixed effects modeling of EGFR mutations in ctDNA as predictor of disease progression in treatment of EGFR-mutant non-small cell lung cancer. Clin Transl Sci. 2022;15:1916–25.
Visal TH, den Hollander P, Cristofanilli M, Mani SA. Circulating tumour cells in the -omics era: how far are we from achieving the ‘singularity’? Br J Cancer. 2022;127:173–84.
Netterberg I, Karlsson MO, Terstappen LWMM, Koopman M, Punt CJA, Friberg LE. Comparing circulating tumor cell counts with dynamic tumor size changes as predictor of overall survival: a quantitative modeling framework. Clin Cancer Res. 2020;26:4892–900.
Mathew M, Zade M, Mezghani N, Patel R, Wang Y, Momen-Heravi F. Extracellular vesicles as biomarkers in cancer immunotherapy. Cancers. 2020;12:2825.
Lin Y, Dong H, Deng W, Lin W, Li K, Xiong X, et al. Evaluation of salivary exosomal chimeric GOLM1-NAA35 RNA as a potential biomarker in esophageal carcinoma. Clin Cancer Res. 2019;25:3035–45.
Mezquita L, Preeshagul I, Auclin E, Saravia D, Hendriks L, Rizvi H, et al. Predicting immunotherapy outcomes under therapy in patients with advanced NSCLC using dNLR and its early dynamics. Eur J Cancer. 2021;151:211–20.
Gavrilov S, et al. Longitudinal tumor size and neutrophil-to-lymphocyte ratio are prognostic biomarkers for overall survival in patients with advanced non-small cell lung cancer treated with durvalumab. CPT Pharmacomet Syst Pharm. 2021;10:67–74.
Benzekri S, Karlsen M, El Kaoutari A, Bruno R, Neubert A, Mercier F, et al. Supporting decision making and early prediction of survival for oncology drug development using a pharmacometrics-machine learning based model. Population Approach Group Europe (PAGE). 2022;Abstr 10276. www.page-meeting.org/?abstract=10276].
Sheiner LB. Learning versus confirming in clinical drug development. Clin Pharm Ther. 1997;61:275–91.
Bruno R, Mercier F, Claret L. Evaluation of tumor size response metrics to predict survival in oncology clinical trials. Clin Pharm Ther. 2014;95:386–93.
Bruno R, Bottino D, de Alwis DP, Fojo AT, Guedj J, Liu C, et al. Progress and opportunities to advance clinical cancer therapeutics using tumor dynamic models. Clin Cancer Res. 2020;26:1787–95.
Kerioui M, Bertrand J, Bruno R, Mercier F, Guedj J, Desmée S. Modelling the association between biomarkers and clinical outcome: An introduction to nonlinear joint models. Br J Clin Pharm. 2022;88:1452–63.
Claret L, Girard P, Hoff PM, Van Cutsem E, Zuideveld KP, Jorga K, et al. Model-based prediction of phase III overall survival in colorectal cancer on the basis of phase II tumor dynamics. J Clin Oncol. 2009;27:4103–8.
Ribba B, Holford NH, Magni P, Troconiz I, Gueorguieva I, Girard P, et al. A review of mixed-effects models of tumor growth and effects of anticancer drug treatment used in population analysis. CPT Pharmacomet Syst Pharm. 2014;3:e113.
Lin RS, Lin J, Roychoudhury S, Anderson KM, Hu T, Huang B, et al. Alternative analysis methods for time to event endpoints under nonproportional hazards: a comparative analysis. Stat Biopharm Res. 2020;12:187–98.
Claret L, Gupta M, Han K, Joshi A, Sarapa N, He J, et al. Evaluation of tumor size response metrics to predict overall survival in Western and Chinese patients with first-line metastatic colorectal cancer. J Clin Oncol. 2013;31:2110–4.
Claret L, Jin JY, Ferté C, Winter H, Girish S, Stroh M, et al. A model of overall survival predicts treatment outcomes with atezolizumab versus chemotherapy in non-small cell lung cancer based on early tumor kinetics. Clin Cancer Res. 2018;24:3292–98.
Chan P, Marchand M, Yoshida K, Vadhavkar S, Wang N, Lin A, et al. Prediction of overall survival in patients across solid tumors following atezolizumab treatments: a tumor growth inhibition-overall survival modeling framework. CPT Pharmacomet Syst Pharm. 2021;10:1171–82.
Madabushi R, Seo P, Zhao L, Tegenge M, Zhu H. Review: role of model-informed drug development approaches in the lifecycle of drug development and regulatory decision-making. Pharm Res. 2022;39:1669–80.
Galluppi GR, Brar S, Caro L, Chen Y, Frey N, Grimm HP, et al. Industrial perspective on the benefits realized from the FDA’s model-informed drug development paired meeting pilot program. Clin Pharm Ther. 2021;110:1172–75.
Shah M, Rahman A, Theoret MR, Pazdur R. The drug-dosing conundrum in oncology—when less is more. N. Engl J Med. 2021;385:1445–47.
Chatelut E, Hendrikx JJMA, Martin J, Ciccolini J, Moes DJAR. Unraveling the complexity of therapeutic drug monitoring for monoclonal antibody therapies to individualize dose in oncology. Pharm Res Perspect. 2021;9:e00757.
Beumer JH, Chu E, Salamone SJ. All optimal dosing roads lead to therapeutic drug monitoring—why take the slow lane. JAMA Oncol. 2022;8:1733–5.
Taylor JMG, Yu M, Sandler HM. Individualized predictions of disease progression following radiation therapy for prostate cancer. J Clin Oncol. 2005;23:816–25.
Sène M, Mg Taylor J, Dignam JJ, Jacqmin-Gadda H, Proust-Lima C. Individualized dynamic prediction of prostate cancer recurrence with and without the initiation of a second treatment: development and validation. Stat Methods Med Res. 2016;25:2972–91.
Krishnan SM, Friberg LE. Bayesian forecasting of tumor size metrics and overall survival. CPT Pharmacomet Syst Pharm. 2022;00:1–10. https://doi.org/10.1002/psp4.12869.
Beyer U, Dejardin D, Meller M, Rufibach K, Burger HU. A multistate model for early decision-making in oncology. Biom J. 2020;62:550–67.
Role of Modelling and Simulation in Regulatory Decision Making in Europe. “https://www.ema.europa.eu/en/documents/presentation/presentation-role-modelling-simulation-regulatory-decision-making-europe_en.pdf; accessed October 14, 2022.
Maitland ML, O’Cearbhaill RE, Gobburu J. Cancer clinical investigators should converge with pharmacometricians. Clin Cancer Res. 2019;25:5182–4.
Mushti SL, Mulkey F, Sridhara R. Evaluation of overall response rate and progression-free survival as potential surrogate endpoints for overall survival in immunotherapy trials. Clin Cancer Res. 2018;24:2268–75.
Burzykowski T, Coart E, Saad ED, Shi Q, Sommeijer DW, Bokemeyer C, et al. Evaluation of continuous tumor-size-based end points as surrogates for overall survival in randomized clinical trials in metastatic colorectal cancer. JAMA Netw Open. 2019;2:e1911750.
Krishnan SM, Friberg LE, Mercier F, Zhang R, Wu B, Jin JY, et al. Multistate pharmacometric model to define the impact of second-line immunotherapies on the survival outcome of IMpower131 study. Clin Pharmacol Ther. 2023; https://doi.org/10.1002/cpt.2838.
Zhou J, Liu Y, Zhang Y, Li Q, Cao Y. Modeling tumor evolutionary dynamics to predict clinical outcomes for patients with metastatic colorectal cancer: a retrospective analysis. Cancer Res. 2020;80:591–601.
Chanu P, Wang X, Li Z, Chen S-C, Samineni D, Susilo M, et al. A disease model for multiple myeloma developed using real world data. PAGE 2021;Abstr 9878. www.page-meeting.org/?abstract=9878.
Chan P, Zhou X, Wang N, Liu Q, Bruno R, Jin YJ. Application of machine learning for tumor growth inhibition—overall survival modeling platform. CPT Pharmacomet Syst Pharm. 2021;10:59–66.
Duda M, Chan P, Bruno R, Jin YJ, Lu J. A pan-indication machine learning (ML) model for tumor growth inhibition—overall survival (TGI-OS) prediction. Clin Pharm Ther. 2021;109:S25.
Laurie M, Lu J. Neural ordinary differential equations for tumor dynamics modeling and overall survival predictions. PAGE 2022;Abstr 9992 www.page-meeting.org/?abstract=9992.
Funding
None.
Author information
Authors and Affiliations
Contributions
RB, PC, MK, FM and KY wrote the manuscript. All authors reviewed and edited the manuscript. All authors approved the version to be published.
Corresponding author
Ethics declarations
Competing interests
All authors but JG are Roche employees and hold Roche stocks. JG declares no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bruno, R., Chanu, P., Kågedal, M. et al. Support to early clinical decisions in drug development and personalised medicine with checkpoint inhibitors using dynamic biomarker-overall survival models. Br J Cancer 129, 1383–1388 (2023). https://doi.org/10.1038/s41416-023-02190-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02190-5
This article is cited by
-
First-line oxaliplatin-based chemotherapy and nivolumab for metastatic microsatellite-stable colorectal cancer—the randomised METIMMOX trial
British Journal of Cancer (2024)
-
Immune check points in cancer treatment: current challenges and perspectives
British Journal of Cancer (2023)
-
External validation of a tumor growth inhibition-overall survival model in non-small-cell lung cancer based on atezolizumab studies using alectinib data
Cancer Chemotherapy and Pharmacology (2023)