Abstract
Lichen planus is a chronic, mucocutaneous inflammatory condition which, due to its prevalence, will be familiar to the dental profession. However, diverse forms of presentation, important differential diagnosis, potential malignant change and monitoring requirements often result in challenges for those in primary care. This paper looks to examine these challenges and provide information to support those who are involved in recognition and management of patients with lichen planus.
Key points
-
Refer any suspected oral lichen planus or lichenoid lesion to specialist services unless competent to manage in practice and if appropriate.
-
Treat symptomatic patients with first line topical corticosteroid preparations for short-term management and as directed by specialist services for long-term management. For lichenoid contact reactions, engage with specialist requests to replace amalgam restorations.
-
General dental practitioners should have awareness of the risk of malignant transformation associated with oral lichen planus, monitor patients and alert specialist services to any clinically significant changes.
Similar content being viewed by others
Introduction
Lichen planus (LP) is a mucocutaneous chronic inflammatory condition which can affect the oral cavity, skin and genitalia, as well as other extra-oral sites. LP affects just under 1% of the general population.1,2 Although it can occur at any age, it predominantly affects adults over 40 years old.2
While oral lichen planus (OLP) is a chronic inflammatory disorder of unknown aetiology, oral lichenoid lesions (OLLs) develop in response to an extrinsic agent.3
Lichenoid inflammation is characteristic of both OLP and OLLs: a band-like infiltration of lymphocytes at the epithelial-connective tissue interface with associated epithelial basal cell damage and saw-tooth-shaped epithelial rete ridges.4 While some clinical and subtle histological differences exist, differentiating between OLP and OLLs can be challenging, which has led to debate (even among specialists) in terminology.5 OLP and OLLs present routinely to oral medicine (OM) departments, but their prevalence means they will also present to primary care, albeit uncommonly. It is important that general dental practitioners (GDPs) have an appropriate level of knowledge regarding the diagnosis and management of OLP and OLLs as patients with these conditions can present with significant challenges.
Professional monitoring is required for OLP and OLLs in view of their status of oral potentially malignant disorders.6 Varied presentations, important differential diagnoses and difficult management often require referral to secondary care.
Aetiology
Oral lichen planus
LP is a disorder of unknown aetiology affecting mucous and serous membranes. In vitro and ex vivo experimental studies suggest that both CD4+ and CD8+ T-cell responses play a role in the pathogenesis.7,8 Aetiology and disease course are likely multifactorial involving genetic and environmental factors.7
LP has been associated with other autoimmune systemic disorders, such as thyroid disease, ulcerative colitis and myasthenia gravis.9,10 Lichenoid inflammation has been reported in paraneoplastic mucosal disease and Good's syndrome.11,12 Associations have been reported with certain liver diseases and hepatitis B vaccination.13 Conflicting findings have been reported with regards to a relationship between LP and hepatitis C but with an overall larger body of literature supporting a positive association between LP and hepatitis C, including a cohort study in 1,557 patients.14,15 On this basis, hepatitis C testing for LP patients has been recommended at a minimum in high-prevalence countries and high-risk patients.14
Oral lichenoid lesions
OLL is an umbrella term used to describe a diverse range of conditions where the clinical or histological presentation is compatible with OLP but not typical.16 Notwithstanding the lack of universally agreed clinicopathological criteria, OLLs broadly include:
-
1.
Lesions caused by a reaction to exogenous substances:
-
Oral lichenoid contact reactions caused by dental materials (in particular amalgam)
-
Lichenoid drug reactions (Table 1)
Table 1 Examples of common systemic drugs associated with lichenoid drug reactions (non-exhaustive list) -
Oral lichenoid contact reactions caused by cinnamon according to a relatively small body of evidence4,17,18
-
-
2.
Conditions mimicking LP such as oral graft versus host disease.
Challenges in presentation
Extra-oral manifestations
Cutaneous lesions are the most common extra-oral presentation of LP and affect around 50% of patients who present initially with oral symptoms. However, patients presenting initially with cutaneous LP are less likely to have oral lesions.21 The most common sites for cutaneous LP are the flexor surface of the wrists (Fig. 1), lower legs/ankles, trunk and lower back.22 Cutaneous LP presents as a pruritic rash of shiny, flat-topped papules with a purple hue and superimposed white lacy lines (‘Wickham striae').
Other extra-oral sites include the genitals, nails, scalp and less commonly, the larynx, oesophagus, cornea and conjunctiva.23,24 Genital lichen planus is seen in up to 57% of women with OLP.25 Vulvovaginal-gingival syndrome is a severe form of lichen planus, often more resistant to first-line treatment, that can result in scarring and strictures (Box 1).26
Diversity of clinical presentation of oral lichen planus
The diversity of clinical presentations of OLP can make clinical recognition challenging.
There are six presentations, which describe different clinical appearances and disease phases.27 A patient can have multiple presentations at any one time:
-
Reticular - a common presentation of white line lacy patterns caused by epithelial hyperkeratosis. Small papules can also be present
-
Atrophic - areas of erythematous mucosa caused by epithelial thinning
-
Ulcerated/erosive - partial or full thickness loss of stratified epithelium presenting as erythematous areas, with or without a yellow surface and non-erosive reticular periphery
-
Plaque-like - homogenous and well-demarcated white patches often affecting the buccal mucosa and dorsum of tongue
-
Bullous - subepithelial blisters, often affecting the gingivae, that rupture to reveal areas of ulceration
-
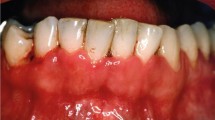
Desquamative gingivitis - a clinical descriptor of inflamed, erythematous, smooth gingivae. OLP is the most common cause of desquamative gingivitis.
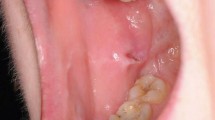
Different clinical presentations in comparison with LP of the lip and amalgam-related OLLs are shown in Figure 2.
The most common presentation of OLP is bilateral, symmetrical, white, reticular striations affecting the buccal mucosa. The tongue and gingivae are the next most common sites.28 Careful consideration is required in cases of desquamative gingivitis and the bullous presentation as these are also features of other immune-mediated mucosal diseases. The palate is rarely involved in OLP and LP-like lesions affecting this site should prompt consideration of an alternative diagnosis for example, lupus. (See ‘other conditions mimicking oral lichen planus' section).29,30
Presentation of oral lichenoid lesions
OLLs can present within the same clinical spectrum as OLP, making differentiation between the two conditions challenging. In the case of lichenoid contact hypersensitivity associated with a dental material such as amalgam, the OLLs are in direct contact with the causative restoration.31 The lesion(s) often resolve or improve on removal of the causative factor, for example, replacement of the amalgam restoration with an alternative material.32
With regards to OLLs induced by medications, a recent review found the mean age of patients with lichenoid drug reactions to be 58.5 years.33 The most frequently reported causative drug were checkpoint inhibitors (anti-PD1/PD-L1 antibodies) and the median time from discontinuing the drug to resolution was 15.7 weeks. Naturally, withdrawal of the culprit drug often requires careful consideration of the risk and benefits and may not always be a practical option, for example, in the context of cancer treatment (Box 2).33
Other conditions mimicking oral lichen planus
Oral lupus erythematosus can present similarly to OLP, with atrophic or erosive patches surrounded by white striations in a ‘sun-ray' appearance. The buccal mucosa, palate and lips are most commonly affected in oral lupus (Fig. 3).16 Oral graft versus host disease presents in patients receiving allogenic stem cell transplants. The clinical appearance and histological characteristics of chronic oral graft versus host disease are indistinguishable from OLP27 (Fig. 4). Both oral lupus erythematosus and graft versus host disease are oral potentially malignant disorders and require surveillance.16
LP-like lesions can be seen in paraneoplastic mucosal disease as described in the S2k guidelines on the management of paraneoplastic autoimmune multiorgan syndrome.34 Suspicion of multi-system disorders should be raised in cases of atypical clinical presentations, and/or constitutional symptoms.
Disease severity and symptoms
While some OLP/OLL cases are asymptomatic, other patients report significant symptoms associated with an adverse impact on quality of life.35 Patients may describe a variety of symptoms (Box 3). Patient-reported symptoms and the impact on daily activities does not necessarily correlate with clinical disease severity. Patient-centered tools for measuring the impact of chronic oral mucosal disease on quality of life have been developed, for example, the Chronic Oral Mucosal Diseases Questionnaires (COMDQ).36
Challenges in diagnosis
Differential diagnoses of suspected OLP and the predominant clinical appearances for each condition are outlined in Box 4.
Biopsy
It has been suggested that a diagnosis of OLP can be made in asymptomatic patients with typical reticular and bilateral buccal striations without the need for a biopsy.28,37 For all other presentations, incisional biopsies are undertaken in secondary care.
The differential diagnosis of oral lichenoid inflammation includes OLP, oral lichenoid reactions, oral lupus erythematosus and chronic graft versus host disease (Box 4). The appearance of the oral lesions, clinical history (including extra-oral symptoms/signs) and results of special investigations are considered together to reach a diagnosis. The role of histopathology in discerning OLP from OLLs can be very limited due to the mostly indistinguishable features. However, subtle histological differences that may be identified include deep perivascular lymphocytic infiltrate in oral lupus, and even in lichenoid reactions.4,38 The value of histopathological review by an oral pathologist should not be overlooked and is recommended by World Health Organisation (WHO) as the gold standard.16
Histopathology is used to distinguish OLP from other immune-mediated mucosal diseases (Box 4). When the clinical presentation is compatible with blistering disorders and/or desquamative gingivitis, the diagnosis must be confirmed with both standard haematoxylin and eosin staining and direct immunofluorescence given the implications on management and prognosis for these conditions. Notably, patients with mucous membrane pemphigoid are referred for ophthalmology review irrespective of history of ocular symptoms due to the potential for subclinical conjunctival involvement and potentially sight-threatening scarring (Box 5).
Patch testing
Lichenoid reactions to dental materials are most commonly seen with amalgam,39 although there are reported cases of lichenoid reactions to acrylics, resins and composites.40 The role of patch testing for suspected OLLs to dental materials has seen conflicting results in the literature. Concerns have been raised about the validity of the test itself in view of differential reactivity between skin and oral mucosa.41 Patch testing should be considered only in conjunction with the clinical presentation. For example, it may prove as a helpful adjunct in patients with an extensively amalgam-restored dentition and widespread lichenoid lesions before embarking on complex and costly dental treatment. On the other hand, patch testing is rarely necessary for lesions in direct contact with an amalgam restoration.39 Access to patch testing in primary care is limited.
Challenges in management
It is recognised that GDPs will have varying degrees of clinical experience with OLP/OLLs and this should be considered when evaluating the appropriateness of a referral to secondary care. The key points of this paper and a more detailed decision-making tool (Fig. 5) are intended to guide GDPs in the referral and management of suspected OLP/OLLs. If any doubt exists, a referral to secondary care should be made.
Malignant transformation
A small proportion of OLP and OLLs demonstrate malignant transformation to oral squamous cell carcinoma (0.5-2.5% over five years).21 The malignant potential of OLP and OLLs has been long debated, but a recent consensus report by the WHO Collaborating Centre for Oral Cancer has listed both OLP and OLLs as potentially malignant disorders.16 OLLs may have a higher transformation rate than idiopathic OLP.42 A systematic review by Giuliani et al. found the overall malignant transformation rate of OLP and OLLs to be approximately 1.37% and 2.43%, respectively.1 Another review found the malignant transformation rate of OLP to be 1.14% and marginally higher for OLLs, but the authors concluded these values were likely underestimations of the true potential of malignant transformation.42 Risk factors for malignant transformation were tongue lesions, smoking, alcohol consumption, atrophic-erosive lesions, hepatitis C infection42 and female sex.1 All patients with OLP/OLLs should be monitored by a dental practitioner. Changes to the typical pattern of disease should instigate a referral to secondary care for consideration of biopsy to assess for dysplasia or malignancy. In cases of suspected malignancy, patients must be referred urgently through the two-week cancer pathway.
Oral lichen planus
Management of OLP is aimed at symptom control, thus minimising impact on daily activities and improving quality of life. Dentists in primary care can initiate first-line treatment for patients with suspected OLP (see ‘first line treatments' section). Assessment of the impact on quality of life is often integral to the risk-benefit analysis of any pharmacological treatment. The impact on quality of life may be measured with validated tools such as the COMDQ.43
Oral lichenoid lesions
OLLs may present atypically when compared to idiopathic OLP and histological diagnosis in secondary care is often required. In the case of a suspected lichenoid reaction to dental materials, the proximity/direct contact of the lesion(s) with the restoration is often sufficient to support a diagnosis without the need for patch testing. Amalgam replacement results in improvement for over 90% of cases of probable lichenoid reactions to amalgam.44 Clinical association between the mucosal lesion and amalgam, together with a positive patch test, was found to be a good predictor of lesion regression.44 On the other hand, some evidence supports the replacement of amalgam with an alternative dental material regardless of patch test findings.45,46,47 Further, restoration replacement may be justified by the higher malignant transformation potential in OLLs compared to OLP, reported by a systematic review.48 A risk-benefit analysis of the risk of malignant transformation versus tooth prognosis post-restorative treatment is required. Lesion resolution is unlikely to occur before three months following restoration replacement.45
Suspected lichenoid drug reactions should be referred to secondary care for diagnostic confirmation and liaison with the prescribing physician regarding drug substitution where appropriate. Drugs from a similar class can be expected to cause a reaction.27
Monitoring
Close surveillance of OLP and OLLs is critical because of the potential for malignant transformation. GDPs are very well-placed to detect early oral cancer. It has been shown that opportunistic screening of high-risk individuals by GDPs may be a cost-effective screening tool for oral cancer.6 There is no universally accepted monitoring interval, although two to four recall appointments per year is deemed a reasonable frequency.37 We suggest that the monitoring interval should be a minimum of six months in primary care. The appearance of mucosal disease should be documented at every appointment, if possible, with supporting clinical photographs.
Monitoring in secondary care is dependent on disease severity. Validated tools, such as the Oral Disease Severity Score, may be used to monitor clinically significant change.49 Primary and secondary care practitioners have a joint role in monitoring and identifying clinical changes that warrant biopsy to exclude dysplasia or malignancy (Box 6).
First line treatments
Asymptomatic OLP/OLLs do not require treatment (other than consideration to removal of suspected trigger in OLLs). Mild symptoms may be controlled by avoidance of substances that exacerbate symptoms, for example, strongly flavoured foods and sodium lauryl sulphate-containing oral hygiene products. Oral hygiene and control of periodontal disease is critical, particularly in patients presenting with desquamative gingivitis.
Where simple conservative measures and topical analgesics are insufficient for symptom control, therapeutic management can be commenced in the form of topical steroids. Topical analgesics and anti-inflammatory treatments suitable for prescribing in primary care are outlined in the Scottish Dental Clinical Effectiveness (SDCEP) guidelines (Table 2 and Table 3, respectively) and the British National Formulary Dental Formulary.
In primary care, hydrocortisone oromucosal tablets, betamethasone mouthwash and beclomethasone inhaler used as an oral spray can be prescribed off-label (Table 3) and should be accompanied by written patient instructions. The SDCEP's Drug prescribing for dentistry guidance includes advice on off-label prescribing. An exemplar patient leaflet for betamethasone mouthwash can be found on the British and Irish Society for Oral Medicine website, together with a general patient information leaflet on OLP.51
Antimicrobial mouthwashes, such as chlorhexidine mouthwash, can be helpful, as oral hygiene adjuncts in cases of highly symptomatic disease interfering with optimal toothbrushing.
Management options in secondary care
In secondary care, alternative topical steroids may be prescribed off-label, including potent/very potent steroid ointments, such as clobetasol or fluocinolone.52 Topical steroids ointments may be applied to the gingivae via custom-made trays. A systematic review found topical betamethasone valerate, clobetasol-17-propionate and fluocinonide to be effective in the treatment of OLP when compared with placebo.53 On the other hand, a Cochrane review did not support superiority of any topical steroid preparation over another.54 There is evidence to support the use of topical calcineurin inhibitors (for example, tacrolimus)55 but with only very low-certainty evidence, suggesting that topical tacrolimus may be superior at resolving pain compared to topical corticosteroids.54
Where adequate disease control is not achieved with topical therapies, systemic therapies are often commenced in secondary care. This includes periodic courses of systemic prednisolone and prophylactic treatments with disease-modifying antirheumatic drugs, such as hydroxychloroquine, azathioprine and mycophenolate mofetil. Patients on anti-proliferative immunosuppressive therapies (for example, azathioprine and mycophenolate mofetil) will be largely managed in secondary care. However, GDPs should be aware of the increased risk of infection and malignancy associated with these medications.
Challenges in referral
GDPs are in the unique position of screening oral mucosal health in the general population. OLP/OLLs are likely to make up only a small proportion of a GDP's patient base. Small patient numbers, diverse presentations, differential diagnoses, risk of malignant change and restricted prescribing options for symptomatic patients makes referral to OM departments an important part of patient care.
Referrals must be via the appropriate local pathway. To reduce delays, GDPs should keep login details updated for any electronic systems. The referral should include a provisional diagnosis of suspected OLP or OLLs supported by relevant findings of a detailed history and examination, such as the suspected trigger of OLLs if appropriate.
Challenges can also arise post-referral as OLP cases are typically not prioritised as urgent. GDPs have a responsibility to manage symptomatic patients in the interim. In the event of symptom interference with oral intake, an explicit request to expedite the referral should be made.
Additionally, OLP patients with stable and low-grade disease are typically discharged back to their GDPs for long-term surveillance and care, with the understanding that re-referral should be made in the event of concerning clinical changes or poor symptom control. This can only be achieved if baseline findings are well-documented/photographed, allowing longitudinal comparisons.
Conclusion
The GDP is ideally placed to screen for and be the first point of contact for patients with OLP and OLLs. Diagnosis and management of these conditions are often complicated by challenges and pitfalls. This paper has highlighted key principles of appropriate management in primary care and appropriate referral to OM departments.
References
Giuliani M, Troiano G, Cordaro M et al. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis 2019; 25: 693-709.
Li C, Tang X, Zheng X et al. Global Prevalence and Incidence Estimates of Oral Lichen Planus: A Systematic Review and Meta-analysis. JAMA Dermatol 2020; 156: 172-181.
Schlosser B J. Lichen planus and lichenoid reactions of the oral mucosa. Dermatol Ther 2010; 23: 251-267.
Müller S. Oral lichenoid lesions: distinguishing the benign from the deadly. Mod Pathol 2017; 30: 54-67.
Carrozzo M, Porter S, Mercadante V, Fedele S. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontol 2000 2019; 80: 105-125.
Speight P M, Palmer S, Moles D R et al. The cost-effectiveness of screening for oral cancer in primary care. In NIHR Health Technology Assessment Programme: Executive Summaries. Southampton: NIHR Journals Library, 2023.
Wenzel J, Tüting T. An IFN-associated cytotoxic cellular immune response against viral, self-, or tumour antigens is a common pathogenetic feature in ‘interface dermatitis'. J Invest Dermatol 2008; 128: 2392-2402.
Ioannides D, Vakirlis E, Kemeny L et al. European S1 guidelines on the management of lichen planus: a cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol 2020; 34: 1403-1414.
Li D, Li J, Li C, Chen Q, Hua H. The Association of Thyroid Disease and Oral Lichen Planus: A Literature Review and Meta-analysis. Front Endocrinol (Lausanne) 2017; 8: 310.
Simon M. Lichen Planus, Lichenoid Eruptions and Cutaneous GraftVersusHost-Reaction. In Hertl M (ed) Autoimmune Diseases of the Skin: Pathogenesis, Diagnosis, Management. Vienna: Springer, 2001.
Pagliaro T, Yau B, Mortimore R, Butler G. Severe oral ulceration with lichen planus-like histology associated with thymoma and delayed antibody detection - A late diagnosis of paraneoplastic pemphigus. Australas J Dermatol 2023; 64: 450-451.
Gubod E R, Ramanathan A, Mohamad Zaini Z, Warnakulasuriya S. Oral Lichen Planus in a Patient With a Thymoma: A Rare Finding. Cureus 2021; 13: e17376.
Tarakji B, Ashok N, Alakeel R et al. Hepatitis B vaccination and associated oral manifestations: a non-systematic review of literature and case reports. Ann Med Health Sci Res 2014; 4: 829-936.
Birkenfeld S, Dreiher J, Weitzman D, Cohen A D. A study on the association with hepatitis B and hepatitis C in 1557 patients with lichen planus. J Eur Acad Dermatol Venereol 2011; 25: 436-440.
Campisi G, Di Fede O, Craxi A, Di Stefano R, Margiotta V. Oral lichen planus, hepatitis C virus, and HIV: no association in a cohort study from an area of high hepatitis C virus endemicity. J Am Acad Dermatol 2004; 51: 364-370.
Warnakulasuriya S, Kujan O, Aguirre-Urizar J M et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis 2021; 27: 1862-1880.
Calapai G, Miroddi M, Mannucci C, Minciullo P, Gangemi S. Oral adverse reactions due to cinnamon-flavoured chewing gums consumption. Oral Dis 2014; 20: 637-643.
Massih M A, Montalli V Â, Araújo V C, Passador-Santos F, Moraes P D. Lichenoid Reactions of The Oral Cavity: a Difficult Diagnosis, Presentation of Two Cases, Amalgam And Cinnamon Lichenoid Reaction. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117: e169.
Teoh L, Moses G, McCullough M J. A review and guide to drug-associated oral adverse effects - Oral mucosal and lichenoid reactions. Part 2. J Oral Pathol Med 2019; 48: 637-646.
González-Moles M Á, Ramos-García P. Oral lichen planus and related lesions. What should we accept based on the available evidence? Oral Dis 2022; 29: 2624-2637.
Robinson M, Hunter K, Pemberton M, Sloan P. Diseases of the oral mucosa. In Robinson M, Hunter K, Pemberton M, Sloan P (eds) Soames' & Southam's Oral Pathology. Oxford: Oxford University Press; 2018.
Wagner G, Rose C, Sachse M M. Clinical variants of lichen planus. J Dtsch Dermatol Ges 2013; 11: 309-319.
Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med 2012; 366: 723-732.
Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal 2014; 2014: 742826.
Belfiore P, Di Fede O, Cabibi D et al. Prevalence of vulval lichen planus in a cohort of women with oral lichen planus: an interdisciplinary study. Br J Dermatol 2006; 155: 994-998.
Setterfield J F, Neill S, Shirlaw P J et al. The vulvovaginal gingival syndrome: a severe subgroup of lichen planus with characteristic clinical features and a novel association with the class II HLA DQB1*0201 allele. J Am Acad Dermatol 2006; 55: 98-113.
Cawson R A. Cawson's Essentials of Oral Pathology and Oral Medicine. Edinburgh: Churchill Livingstone, 2002.
Gururaj N, Hasinidevi P, Janani V, Divynadaniel T. Diagnosis and management of oral lichen planus - Review. J Oral Maxillofac Pathol 2021; 25: 383-393.
Manfredini M, Pedroni G, Bigi L et al. Acquired White Oral Lesions with Specific Patterns: Oral Lichen Planus and Lupus Erythematosus. Dermatol Pract Concept 2021; 11: e2021074.
Mollaoglu N. Oral lichen planus: a review. Br J Oral Maxillofac Surg 2000; 38: 370-377.
Al-Hashimi I, Schifter M, Lockhart P B et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 25.
Issa Y, Brunton P A, Glenny A M, Duxbury A J. Healing of oral lichenoid lesions after replacing amalgam restorations: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004; 98: 553-565.
Maul J-T, Guillet C, Oschmann A et al. Cutaneous lichenoid drug eruptions: A narrative review evaluating demographics, clinical features and culprit medications. J Eur Acad Dermatol Venereol 2023; 37: 965-975.
Antiga E, Bech R, Maglie R et al. S2k guidelines on the management of paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome initiated by the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol 2023; 37: 1118-1134.
Yuwanati M, Gondivkar S, Sarode S C et al. Impact of Oral Lichen Planus on Oral Health-Related Quality of Life: A Systematic Review and Meta-Analysis. Clin Pract 2021; 11: 272-286.
Ni Riordain R, Hodgson T, Porter S, Fedele S. Validity and reliability of the Chronic Oral Mucosal Diseases Questionnaire in a UK population. J Oral Pathol Med 2016; 45: 613-616.
Scully C, Beyli M, Ferreiro M C et al. update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med 1998; 9: 86-122.
Cheng Y S, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 122: 332-354.
Fletcher R, Harrison W, Crighton A. Dental material allergies and oral soft tissue reactions. Br Dent J 2022; 232: 620-625.
Moore M M, Burke F J, Felix D H. Allergy to a common component of resin-bonding systems: a case report. Dent Update 2000; 27: 432-434.
Holmstrup P. Reactions of the oral mucosa related to silver amalgam: a review. J Oral Pathol Med 1991; 20: 1-7.
González-Moles M Á, Ruiz-Ávila I, González-Ruiz L, Ayén Á, Gil-Montoya J A, Ramos-García P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol 2019; 96: 121-130.
Ni Riordain R, Meaney S, McCreary C. A patient-centered approach to developing a qualityoflife questionnaire for chronic oral mucosal diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 111: 578-586.
Thornhill M H, Pemberton M N, Simmons R K, Theaker E D. Amalgam-contact hypersensitivity lesions and oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2003; 95: 291-299.
Issa Y, Duxbury A J, Macfarlane T V, Brunton P A. Oral lichenoid lesions related to dental restorative materials. Br Dent J 2005; 198: 361-366.
Dunsche A, Kästel I, Terheyden H, Springer I N, Christophers E, Brasch J. Oral lichenoid reactions associated with amalgam: improvement after amalgam removal. Br J Dermatol 2003; 148: 70-76.
Rahat S, Kashetsky N, Bagit A et al. Can We Separate Oral Lichen Planus from Allergic Contact Dermatitis and Should We Patch Test? A Systematic Review of Chronic Oral Lichenoid Lesions. Dermatitis 2021; 32: 144-150.
Fitzpatrick S G, Hirsch S A, Gordon S C. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc 2014; 145: 45-56.
Ormond M, McParland H, Thakrar P et al. Validation of an Oral Disease Severity Score for use in oral lichen planus. Br J Dermatol 2022; 186: 1045-1047.
Scottish Dental Clinical Effectiveness Programme. Drug prescribing. 2021. Available at https://www.sdcep.org.uk/published-guidance/drug-prescribing/ (accessed July 2023).
British and Irish Society for Oral Medicine. Homepage. Available at https://bisom.org.uk/ (accessed July 2023).
Rudralingam M, Randall C, Mighell A J. The use of topical steroid preparations in oral medicine in the UK. Br Dent J 2017; 223: 633-638.
Davari P, Hsiao H-H, Fazel N. Mucosal lichen planus: an evidence-based treatment update. Am J Clin Dermatol 2014; 15: 181-195.
Lodi G, Manfredi M, Mercadante V, Murphy R, Carrozzo M. Interventions for treating oral lichen planus: corticosteroid therapies. Cochrane Database Syst Rev 2020; DOI: 10.1002/14651858.CD001168.pub3.
Sotoodian B, Lo J, Lin A. Efficacy of Topical Calcineurin Inhibitors in Oral Lichen Planus. J Cutan Med Surg 2015; 19: 539-545.
Acknowledgements
The authors would like to thank Helen McParland, Consultant in Oral Medicine, Guy's and St Thomas' NHS Foundation Trust and Daniel Finn, Consultant in Oral Medicine, Liverpool University Dental School, for their help sourcing images included in this paper.
Author information
Authors and Affiliations
Contributions
Rebecca Binnie, Marianne Louise Dobson, Anna Chrystal and Karolin Hijazi contributed equally to every stage in the production of this paper.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0.© The Author(s) 2024.
About this article
Cite this article
Binnie, R., Dobson, M., Chrystal, A. et al. Oral lichen planus and lichenoid lesions - challenges and pitfalls for the general dental practitioner. Br Dent J 236, 285–292 (2024). https://doi.org/10.1038/s41415-024-7063-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-024-7063-y