Abstract
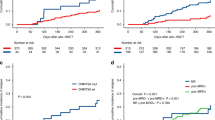
Early relapse (ER) following Autologous Hematopoietic Cell Transplantation (AHCT) confers a poor prognosis. We therefore developed a novel scoring system to predict ER. A total of 14,367 AHCT-1 patients were transplanted between 2014 and 2019, and were conditioned with Melphalan 200 mg/m2 (Mel200) (n = 7228; 2014–2017) (training cohort); Mel200 (n = 5616; 2018–2019) or Mel140 (n = 1523; 2018–2019) (validation cohorts). PFS-12 and the Cumulative Incidence of Relapse at 12 months were 84.1% and 14.7% (training Mel200), 87.2% and 11.6% (validation Mel200), and 80.3% and 16.9% (validation Mel140), respectively. The points in the risk score were: 0, 1,2 for ISS stages I, II, and III; Disease status: 0 (CR/VGPR); 1 (PR); 2 (SD/MR); 4 (Relapse/Progression); and 1 for Karnofsky ≤ 70. The distribution of scores: 0 (24%), 1 (33.9%), 2 (29.6 %), 3 (9.5%), and ≥4 (2.7%). The score separated PFS-12, with the lowest risk group (n = 1752) having a PFS-12 of 91.7% and the highest risk group (n = 195) 57.1%. This also applied in cytogenetically high-risk patients. If the pre-score baseline risks are 15% (standard risk) and 25% (high-risk), a score of ≥4 confers calculated risks of 38% and 54%, respectively. This novel EBMT ER score, therefore, allows for the identification of five discrete prognostic groups.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The EBMT database is not open to general access per EBMT regulations.
Change history
14 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41409-023-02014-3
References
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib–melphalan–prednisone, with or without bortezomib–lenalidomide–dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020;7:e456–68.
Stadtmauer EA, Pasquini MC, Blackwell B, Hari P, Bashey A, Devine S, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: results of the BMT CTN 0702 Trial. J Clin Oncol. 2019;37:589–97.
Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:309–22.
Dhakal B, Shah N, Kansagra A, Kumar A, Lonial S, Garfall A, et al. ASTCT clinical practice recommendations for transplantation and cellular therapies in multiple myeloma. Transpl Cell Ther. 2022;28:284–93.
Kumar S, Mahmood ST, Lacy MQ, Diapenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transpl. 2008;42:413–20.
Kastritis E, Roussou M, Eleutherakis-Papaiakovou E, Gavriatopoulou M, Migkou M, Gika D, et al. Early relapse after autologous transplant is associated with very poor survival and identifies an ultra-high-risk group of patients with myeloma. Clin Lymphoma Myeloma Leuk. 2020;20:445–52.
Corre J, Montes L, Martin E, Perrot A, Caillot D, Leleu X, et al. Early relapse after autologous transplant for myeloma is associated with poor survival regardless of cytogenetic risk. Haematologica 2020;105:e480–3.
Bygrave C, Pawlyn C, Davies F, Craig Z, Cairns D, Hockaday A, et al. Early relapse after high‐dose melphalan autologous stem cell transplant predicts inferior survival and is associated with high disease burden and genetically high‐risk disease in multiple myeloma. Br J Haematol. 2020;193:551–5.
Lahuerta JJ, Paiva B, Vidriales MB, Cordon L, Cedena MT, Puig N, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–10.
Chakraborty R, Muchtar E, Kumar SK, Jevremovic D, Buadi FK, Dingli D, et al. Impact of post-transplant response and minimal residual disease on survival in myeloma with high-risk cytogenetics. Biol Blood Marrow Transpl. 2017;23:598–605.
Dhakal B, D’Souza A, Callander N, Chhabra S, Fraser R, Davila O, et al. Novel prognostic scoring system for autologous hematopoietic cell transplantation in multiple myeloma. Br J Haematol. 2020;191:442–52.
Zaccaria GM, Bertamini L, Petrucci MT, Offidani M, Corradini P, Capra A, et al. Development and validation of a simplified score to predict early relapse in newly diagnosed multiple myeloma in a pooled dataset of 2190 patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27:3695–703.
Harrell, FE (ed) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. 2nd ed. Springer-Verlag, New York, 2001.
Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–44.
Auner HW, Iacobelli S, Sbianchi G, Knol-Bout C, Blaise D, Russell NH, et al. Melphalan 140 mg/m2 or 200 mg/m2 for autologous transplantation in myeloma: results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT Chronic Malignancies Working Party. Haematologica 2017;103:514–21.
Cerchione C, Usmani SZ, Stewart AK, Kaiser M, Rasche L, Kortüm M, et al. Gene expression profiling in multiple myeloma: redefining the paradigm of risk-adapted treatment. Front Oncol 2022;12:820768.
Jethava Y, Mitchell A, Zangari M, Waheed S, Schinke C, Thanendrarajan S, et al. Dose-dense and less dose-intense Total Therapy 5 for gene expression profiling-defined high-risk multiple myeloma. Blood Cancer J. 2016;6:e453–e453.
Karam D, Kumar S. Post-transplant maintenance treatment options in multiple myeloma. Oncol Ther. 2021;9:69–88.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomized, open-label, phase 3 study. Lancet 2019;394:29–38.
Van de Donk NWCJ, Agha M, Cohen A, Cohen YC, Anguille S, Kerre T, et al. Biological correlative analyses and updated clinical data of ciltacabtagene autoleucel (cilta-cel), a BCMA-directed CAR-T cell therapy, in patients with multiple myeloma (MM) and early relapse after initial therapy: CARTITUDE-2, cohort B. J Clin Oncol. 2022;40:8029.
Acknowledgements
The study has been performed within the EBMT database utilizing EBMT resources. SI has performed the statistical analysis that led to this novel ER risk-calculating model.
Author information
Authors and Affiliations
Contributions
MB planned the study and wrote the manuscript, LK prepared the study-specific EBMT database which was presented to SI who performed the statistical analysis and modeled the early relapse score; SI, PH, and IYA contributed to the manuscript preparation; all authors contributed to data enrollment and revised manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The affiliations for Dr Claudia Sossa were incomplete and has now been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beksac, M., Iacobelli, S., Koster, L. et al. An early post-transplant relapse prediction score in multiple myeloma: a large cohort study from the chronic malignancies working party of EBMT. Bone Marrow Transplant 58, 916–923 (2023). https://doi.org/10.1038/s41409-023-01999-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01999-1