Abstract
Objective
The search for an osteopromotive material that enhances the efficacy of alloplasts in reconstructive surgeries has been going on for years. This study aimed to histologically and histomorphometrically evaluate the efficiency of Moringa oleifera leaf extract as an osteopromotive biomaterial.
Design
The study is a prospective randomized controlled animal study. 24 adult male New Zealand rabbits were equally allocated into test and control groups. Critical-sized bone defects were created in the edentulous areas of the mandibles of rabbits. The defects of the control group were filled with Beta-tricalcium Phosphate, while the defects of the test group were filled with Beta-tricalcium Phosphate combined with Moringa oleifera leaf extract. The results were evaluated histologically and histomorphometrically.
Results
Histological and histomorphometric analysis showed a significant increase in the surface area of bone and the number of osteoblasts in test groups compared to those in the control groups.
Conclusion
Moringa oleifera leaf extract has a positive effect on bone regeneration in critical-sized bone defects.
Similar content being viewed by others
Introduction
Periodontal disease includes a broad spectrum of symptoms and manifestations, involving hard and soft tissues, that oftentimes acts as a cascade eventually leading to bone loss [1]. The mandible is a bone that is actively integral in speech and mastication and passively maintains the shape of the face [2]. Large mandibular defects may result from trauma, infection, tumors managed with resection or periodontitis [3]. Large defects that wouldn’t heal on their own present a challenge to clinicians all over the world.
A critical-sized defect is the smallest defect that won’t spontaneously heal on its own through the duration of the experiment nor through the animal’s lifetime [4].
As bone defects greatly affect mastication, and occasionally esthetics, slow regeneration could impose a therapeutic problem. One of the methods to overcome this problem is combining bone graft with osteopromotive components that enhance new bone formation such as herbal extracts [5]
Moringa oleifera (MO) leaves contain various flavonoids that can induce osteogenic differentiation of mesenchymal stem cells derived from bone marrow [6]. Flavonoids protect cells from oxidative stress by scavenging free radicals [7]. Moringa oleifera leaves contain antibacterial phytochemicals as Pterygospermin, Benzyl Isothiocyanate and Benzyl Glucosinolate [8]
Moringa oleifera has been widely tested as an oral care product in humans. One study was conducted to assess the effect of Moringa oleifera leaf extract in patients suffering from Early Childhood Caries. It was found that gargling with Moringa oleifera leaf extract effectively diminishes plaque formation [9]
A study conducted by Patel et al. [10] has proved that flavonoids and other components in Moringa oleifera have positive osteoblastic stimulating potential. A study conducted by Djais et al. [11] evaluating the effect of combining Moringa oleifera with Demineralized Freeze Dried Dentin Matrix (DFDDM) in socket preservation suggested that the combination can effectively generate fibroblast and osteoblast expressions. Moringa oleifera effectively regulates the p38α/MAPK14-OPG/RANKL pathway [12]. It does not only alter the expression of inflammatory cytokines, but Moringa oleifera leaf extract also substantially reduces alveolar bone resorption [12]
Pure β-TCP is a popular choice for monitoring healing due to its availability and ease of handling [13]. A systematic review and meta-analysis published in 2021 that included 5 studies showed that β-TCP used to manage infrabony defects resulted in positive outcomes regarding bone fill and gain of clinical attachment. However, when used with growth factors, β-TCP showed superior results to those shown when used alone [14]
Animal models allow researchers to histologically assess the processes of bone remodeling and regeneration on a cellular level to understand the course of healing and the effect of regenerative materials. The results of this study could contribute to a better quality of life for thousands of people using a biocompatible, cost-effective and widely available material that could enhance the healing process of bone.
In our study, the effect of combining β-TCP with Moringa oleifera leaves extract on healing of critical sized bone defects in rabbits was assessed.
Materials and methods
Study design and sample size
This study is a prospective randomized controlled animal study. The authors followed ARIVE guidelines 2.0.
Sample size was estimated assuming alpha error = 5% and study power = 80%. Djais et al. [11] reported mean ± SD osteoblasts number after 21 days = 90.08 ± 8.07 when moringa was used, and 113.3 ± 12.50 when moringa was combined with freeze dried dentin matrix graft Fig. 1. Based on comparison of means, sample size was calculated to be 5 per group, increased to 6 to make up for laboratory processing errors. The total sample size = number of groups × number per group × number of timepoints = 2 × 6 × 2 = 24.
Ethical statement
The study was performed after gaining the approval of the Research Ethics Committee, Faculty of Dentistry, Alexandria University.
All animals’ procedures followed the National Research Council Guidelines for the care and use of laboratory animals [15].
Randomization and experimental procedure
Random allocation was done, by using a computer-generated random sequence of numbers to assign treatment status to decrease the risk of confounding. Randomization was conducted by giving each defect used in this study a number from 1 to 24 and using computer assisted software; 12 rabbits were randomly allocated to each of the 2 groups. Half of each group were euthanized after 4 weeks, and the other half after 8 weeks.
Preparation of the aqueous extract of Moringa oleifera leaves
Fresh Moringa oleifera leaves were left to dry for 7 days, then infused in distilled water (100 glL). The mixture was then boiled for 20 min then filtered out [16].
24 adult male New Zealand rabbits of 6–7 months of age, weighing 2.5–3.5 kgs were supplied from the national institute of research.
General anesthesia was induced by an intramuscular injection of a combination of 25 mg/kg weight ketamine and 5 mg/kg body weight xylazine.
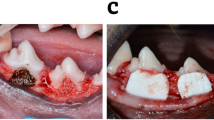
The edentulous alveolar ridge on the right side of the body of the mandible between the incisor and the first posterior tooth of each animal was selected for the surgical site. A full mucoperiosteal flap was raised intraorally. Osseous defects measuring 6 mm (mesiodistal) ×4 mm (buccolingual) ×3 mm (apicocoronally) were prepared in the edentulous diastema [17, 18] (Fig. 2).
The osseous defects were washed out with sterile saline to remove any bone particles that could possibly initiate osteoinduction [19]. Defects were filled with β-TCP in the control group, and filled with a combination of β-TCP and Moringa oleifera leaves extract in the test group.
Flaps were then repositioned and sutured with 3-0 black silk suture. To control postoperative swelling and pain, a subcutaneous injection of 4 mg/kg Carprofen injection was administered twice daily for four consecutive days.
Animals were euthanized using an overdose intravenous injection of pentobarbital 120 mg/kg (Streuli Pharma AG, Uznach, Switzerland) [20].Disposal of animals was done through incineration.
Blinding
Concealment of group allocation from the researcher performing the histological examination ensured a single-blind study and made the results of the study less likely to be biased.
Outcome measures
Histological assessment
Bony specimens were fixed with 10% freshly prepared formalin for 24 h then decalcified using 10% formic acid for 7 days. Embedding was done using paraffin wax; bone blocks were sectioned by microtome for serial sections of 4 μm. Sections were placed on slides and stained using hematoxylin and eosin (H&E) stain, and Trichrome stain. The morphological characters of the newly formed bone were evaluated.
Histomorphometric assessment
10 images at ×100 magnification and 10 images at ×200 magnification were taken for every specimen under light microscope.
The following variables were assessed using Image analysis software J 1.46r [21] software:
-
1.
The percentage of the surface area of newly formed bone compared to the total surface area of the surgically induced critical sized defect.
-
2.
Osteoblastic count: the number of osteoblasts in each photomicrograph was counted.
Statistical methods
Descriptive statistics were calculated as means, standard deviation (SD) and range. Normality was checked using descriptive statistics, plots (Q-Q plots and histograms), and Shapiro Wilk normality test. All variables showed normal distribution, so parametric tests were used. For normally distributed data, comparison between two independent population were done using independent t-test, while the comparison between the same group at a different period of time was done using paired t-test.
The significance level was set at p value < 0.05. Data were analyzed using IBM SPSS software package (Version 24.0).
Results
Histological results
Four-week follow up results
Four weeks postoperatively, the surgical defects of the control group were filled with bone trabeculae of moderate thickness and amount (Fig. 3). Numerous blood vessels containing red corpuscles of red blood cells were found in the marrow spaces surrounding the newly formed bone trabeculae.
A Control group showing numerous blood vessels containing red corpuscles of red blood cells are found in the marrow spaces surrounding the newly formed bone trabeculae. (H&E; original magnification ×100). B Test group shows a huge amount of blood vessels with marrow spaces surrounding the trabeculae of the newly formed bone (H&E; original magnification ×100). C Control group light micrograph showing mature bone within defect with wide marrow spaces of moderate vascularity (Masson’s trichrome; original magnification ×100). D Test group light micrograph showing newly formed bone lined by active osteoblast. Fibrous mapping of newly formed trabeculae is also noticed. (Masson’s trichrome; original magnification ×100).
In the test group, the entire defect was filled with bone trabeculae of greater amount and thickness than the control group. Primary osteons were clearly visible in various areas within the newly formed bone lined by a dense layer of voluminous osteoblasts and surrounded by highly vascular marrow spaces. Also, reversal lines were clearly seen within the newly formed bone trabeculae.
Trichrome stained sections demonstrating the histological outcomes at 4 weeks time point show interconnected trabeculae formation of different sizes of immature bone separated by considerable amounts of Beta Tricalcium Phosphate for the control group. Greater amount of bone is formed in the test group than in the control group. The formed trabeculae are thicker and more inter-communicated. Considerable blood supply is also seen between intervening connective tissue.
Eight-week follow up results
After eight weeks, the surgical defects of the control group are filled with bone trabeculae of various thickness and maturity. The formed bone enclosed particles of bone grafts and is surrounded by dense fibrous marrow spaces.
The test group shows inter-communicated dense bone trabeculae filling the entire surgical defect. Remnants of bone graft particles are seen within bone trabeculae lined by numerous plump osteoblasts and enclose numerous osteocytes (Fig. 4A, B).
A Control group shows the intermingle between graft particles (arrows) and the formed mature bone. (H&E; original magnification ×100). B Test group shows the newly formed bone that is lined by active plump osteoblasts (thick arrow) and contains numerous osteocytes (thin arrows). (H&E; original magnification ×100). C Light micrograph of the control group showing bone trabeculae within defect with marrow spaces of moderate vascularity (Masson’s trichrome; original magnification ×100). D Light micrograph of the test group showing mature bone within the defect, the marrow spaced show evident fibrous tissue and moderate vascularity. (Masson’s trichrome; original magnification ×100).
Figure. 4C, D shows trichrome stained sections demonstrating the histological outcomes at 8 week-time point. The control group shows thin bone trabeculae that are not interconnected and separated by broader surface area of intervening connective tissue. The test group resulted in greater amounts of intercommunicated bone trabeculae that are thicker than those presented in the control groups.
Histomorphometric analysis
The percentage of newly formed bone in relation to the total surface area of the defect was calculated in all groups. The mean percentage of newly formed bone is illustrated in Table 1. After four weeks, the mean percentage of surface area of bone formed was 19.42% in the control group and 31.05% in the test group, yielding a significant difference between both groups with a 0.001 p-value. Likewise, the results studied at 8-week time point resulted in a significant difference with a 0.028 p-value between the test and control groups as the mean percentage of surface area of bone was 33.78% for the control group and 41.12% for the test group.
The graph in Fig. 5 summarizes the results of our study by demonstrating the mean percentage of surface area of bone formed in relation to the total surface area of the defect.
After 1 month, the range of percentage of surface area of bone in comparison to the total surface area of the defect in the control group ranges between 16.77% and 23.36% with a mean of 19.42%, while in the test group the percentage ranged from 27.72% to 38.34%. After 2 months, the range of percentage of surface area of bone in comparison to the total surface area is from 25.07% to 39.92% with a mean of 33.78% for the control group while for the test group the percentage ranged from 33.34% to 44.99% with a mean of 41.12%.
The osteoblastic count was studied by quantitative assessment of the histologic photomicrographs of all study groups using image J software, as represented in Table 2. After 4 weeks, the mean number of osteoblasts per photomicrograph was 77 for the control group and 101 for the test group, yielding a significant difference between both groups with a 0.001 p-value. Likewise, after 8 weeks, the mean number of osteoblasts for the control group was 61.71 and 70.43 for the test group, yielding a significant difference between both groups with a 0.003 p-value.
The graph in Fig. 6 demonstrates the mean number of osteoblasts counted per photomicrograph for all study groups.
The figure shows that the range of number of osteoblasts per photomicrograph for the control group after 1-month ranges from 69 to 81 with a mean of 77 osteoblasts while the number of osteoblasts per photomicrograph for the test group after 1 month ranged from 97 to 108 with a mean of 101.86. After 2 months, the number of osteoblasts per photomicrograph for the control group ranged from 56 to 68 with a mean of 61.71 while for the test group it ranged from 67 to 75 with a mean of 70.43 osteoblasts per photomicrograph.
Discussion
In the current study, we investigated the effect of Moringa oleifera, a plant-based traditional medicine, in the management of critical-sized bone defects in the mandibles of rabbits.
We chose rabbits as they are considered the most convenient small animal that allows histological examination of bone regeneration for multiple reasons. The first reason is that the mandibles of rabbits are larger than those of rats, making the surgical procedure technically more feasible and reproducible [22]. More importantly, rabbits manifest multicellular unit remodeling highly similar to the remodeling that occurs in humans [22].
Most studies assess periodontal regenerative therapy through clinical and radiographic evaluation. However, we chose to conduct a histological and histomorphometric experimental study. This is the first study to assess the effect of Moringa oleifera leaf extract in combination with Beta-tricalcium Phosphate in critical-sized intra-oral bone defects. The monocortical critical-sized defects performed in this study allowed us to examine the direct effect of Moringa oleifera leaf extract on bone filling in a simple and standardized manner [23].
Histomorphometry is the quantitative assessment of histological samples. It has long been proven to be a powerfully valid tool widely used to assess bone regeneration [24].
Our study revealed that Moringa oleifera leaf extract substantially increased the percentage of the surface area of bone in relation to the total surface area of the surgically induced critical-sized defect. The results of our study are consistent with the results of a study held in 2019, in which the expression of osteocalcin and Transforming Growth Factor Beta1 (TGF-β1) was studied. The participants of this study were divided into groups; all of which underwent extraction of the left mandibular incisor. The mean values for the expression of osteocalcin and TGF-β1 were higher in the groups treated with Moringa oleifera leaf extract, Demineralized Freeze Dried Bovine Bone Xenografts (DFDBBX) and Polyethylene glycol (PEG) than for the groups treated with DFDBBX and PEG alone [25] One reason for these results may be attributed to is the myriad amount of saponins and flavonoids found in Moringa oleifera.
In 2008, an in vitro study assessed the activity of Moringa oleifera leaf extract as a free radical scavenger and concluded that it has a potential therapeutic antioxidant effect [26]. Moringa oleifera has also been showing anti-inflammatory effects by reducing the production of Interleukin-6 (IL-6), a pro-inflammatory cytokine induced by Porphyromonas gingivalis [27].
We also found that the addition of Moringa oleifera leaf extract to alloplastic bone graft significantly increased the number of osteoblasts in the defect, especially in the proliferative stage. These results come in accordance with a study held by Jeong et al that found that saponins have an in vitro osteogenic action that affects osteoblastic proliferation and differentiation [28]. Flavonoids in Moringa oleifera play an important role in bone repair, but kaempferol flavonoids have been proven to be of special importance. Kaempferol activates estrogen receptors inducing osteoblasts differentiation [29].
Moringa leaves possess a considerably high amount of tannins ranging from 13.2 to 20.6 g tannin/kg of dried leaves [30]. These tannins can inhibit osteoclast differentiation, which in turn facilitates new bone formation [31].
In 2020, research was conducted to investigate the effect of Moringa oleifera leaf extract on orthodontic tooth movement after its administration in tension areas. It was concluded that Moringa oleifera leaf extract substantially increased the number of osteoblasts and decreased the number of osteoclasts in tension areas where it was administered [32].
Another study was held by Khan et al. [33] in 2022 in which the effect of Moringa oleifera leaf extract on osteoblast cell differentiation was examined. This study used Alkaline phosphatase analysis, which concluded that Moringa oleifera leaf extract increases osteoblast cell differentiation and enhances the expression of both Runt-related transcription factor 2 (RUNX2) and Bone morphogenetic protein 2 (BMP2) genes when used in a concentration of 25 or 50 μg /mL. Marupanthorn et al. [34] conducted a study that demonstrated that Moringa oleifera leaf extract potentiates osteogenic differentiation of bone marrow-derived stem cells in pigs.
Among the limitations of our study is the lack of longer follow-up periods, and that the study was conducted on a small sized animal. In the future, it is recommended to test the efficacy of Moringa oleifera leaf extract on bone healing on larger sample sizes of bigger sized animals and in randomized controlled clinical trials. The use of the critical sized defect model in this experiment ensures the reliability of results and the standardization of the surgical procedures. This study introduces a natural osteopromotive biomaterial that could enhance the quality of regenerated bone in a simple and efficient manner.
Conclusion
Our findings showed that Moringa oleifera leaf extract has a positive effect on the quality and quantity of bone formed. The significant increase in the percentage of surface area of bone formed in test group compared to the size of the defect paves the way for further clinical research that could revolutionize the field of bone regeneration through a cost-effective, easily available osteopromotive material.
Moringa oleifera has been used in animal and clinical studies and has shown no side effects [35]. Animal studies testing the efficiency of Moringa oleifera on bone regeneration are recommended using large animal models, and in combination with different bone grafting materials.
Data availability
The datasets used during the current study are available from the corresponding author upon reasonable request.
References
Cochran DL. Inflammation and Bone Loss in Periodontal Disease. J Periodont. 2008;79:1569–76.
Koca CG, Kösehasanoğulları M. Evaluation of single-dose applied teriparatide effect on bone healing with histomorphometric and micro-ct analysis. J Cranio Maxillofac Surg. 2021;49:98–103.
Keating JF, Simpson A, Robinson C. The management of fractures with bone loss. J Bone Jt Surg Br Vol. 2005;87:142–50.
Ashour AA, Zaghloul M, Mahmoud W, Helal ME, Grawish ME. Gelfoam haemostatic agent with or without autologous bone marrow-derived stem cells for the regeneration of critical-size mandibular defects in the rabbit. Int J Oral Maxillofac Surg. 2018;47:1488–94.
Lee D-H, Kim I-K, Cho H-Y, Seo J-H, Jang J-M, Kim J. Effect of herbal extracts on bone regeneration in a rat calvaria defect model and screening system. J Korean Assoc Oral Maxillofac Surg. 2018;44:79–85.
Al-Azzawi AS, Al-Ghaban NMH. Histomorphometric Evaluation of the Effect of Local Application of Moringa Oliefera/Marine Collagen on Bone Healing in Rats. J Res Med Dent Sci. 2021;9:225–30.
YOKOMIZO A, MORIWAKI M. Effects of Uptake of Flavonoids on Oxidative Stress Induced by Hydrogen Peroxide in Human Intestinal Caco-2 Cells. Biosci Biotechnol Biochem. 2006;70:1317–24.
Owolabi J, Opoola E, Caxton-Martins E. Healing and Prophylactic Effects of Moringa oleifera Leaf Extract on Lead Induced Damage to Haematological and Bone Marrow Elements in Adult Wistar Rat Models. J Aquac Res Dev. 2012;1, https://doi.org/10.4172/scientificreports.386.
Fajriani I. The effectiveness of gargle contain extract of Moringa oleifera leaves to inhibit plaque formation on early childhood caries (ECC). Int J Appl Pharm. 2019;11:46–9.
Patel C, Rangrez A, Parikh P. The anti-osteoporotic effect of Moringa oliefera on osteoblastic cells: SaOS 2. IOSR J Pharm Biol Sci. 2013;5:10–7.
Djais AI, Oktawati S, Thahir H, Hatta M, Sukmana BI, Dewi N, et al. The Effect of the Combination of Demineralization Freeze Dried Dentin Matrix (DFDDM) and Moringa oleifera Lam to increase Fibroblas and Osteoblas cell in Alveolar Bone after Caviacobaya? s Tooth Extraction. Syst Rev Pharmacy. 2020;11:523–30.
Wang F, Long S, Zhang J. Moringa oleifera Lam. leaf extract safely inhibits periodontitis by regulating the expression of p38α/MAPK14-OPG/RANKL. Arch Oral Biol. 2021;132:105280.
Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am. 2007;19:513–21.
Jasser RAL, AlSubaie A, AlShehri F. Effectiveness of beta-tricalcium phosphate in comparison with other materials in treating periodontal infra-bony defects around natural teeth: a systematic review and meta-analysis. BMC Oral Health. 2021;21:219.
Care Gft, Animals UoL. National research council (US) committee for the update of the guide for the care and use of laboratory animals. Washington, DC, USA: National Academies Press; 2011.
Moichela FT, Adefolaju GA, Henkel RR, Opuwari, CS. Aqueous leaf extract of Moringa oleifera reduced intracellular ROS production, DNA fragmentation and acrosome reaction in Human spermatozoa in vitro. Andrologia. 2021;53:e13903.
Shanbhag S, Pandis N, Mustafa K, Nyengaard JR, Stavropoulos A. Alveolar bone tissue engineering in critical-size defects of experimental animal models: a systematic review and meta-analysis. J Tissue Eng Regen Med. 2017;11:2935–49.
Park J-B, Lee K, Lee W, Kim H, Lee K, Kim I. Establishment of the chronic bone defect model in experimental model mandible and evaluation of the efficacy of the mesenchymal stem cells in enhancing bone regeneration. Tissue Eng Regen Med. 2013;10:18–24.
Olmedo ML, Landry PS, Sadasivan KK, Albright JA, Meek WD, Routh R, et al. Regulation of Osteoblast Levels During Bone Healing. J Orthop Trauma. 1999;13:356–62.
Schaller B, Fujioka-Kobayashi M, Zihlmann C, Schuler VC, Katagiri H, Lang NP, et al. Effects of additional collagen in biphasic calcium phosphates: A study in a rabbit calvaria. Clin Oral Investig. 2020;24:3093–103.
Nanes BA. Slide Set: Reproducible image analysis and batch processing with ImageJ. BioTechniques. 2015;59:269–78.
Baskin JZ, White BM, Vasanji A, Love TE, Eppell SJ. Mandible biomechanics and continuously erupting teeth: a new defect model for studying load-bearing biomaterials. Biomedicines. 2021;9:730.
Schlegel KA, Lang FJ, Donath K, Kulow JT, Wiltfang J. The monocortical critical size bone defect as an alternative experimental model in testing bone substitute materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006;102:7–13.
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units: report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610.
Kresnoadi U, Rahmania PN, Caesar HU, Djulaeha E, Agustono B, Ari MDA. The role of the combination of Moringa oleifera leaf extract and demineralized freeze-dried bovine bone xenograft (xenograft) as tooth extraction socket preservation materials on osteocalcin and transforming growth factor-beta 1 expressions in alveolar bone of Cavia cobaya. J Indian Prosthodontic Soc. 2019;19:120.
Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-Ngam L, et al. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol. 2008;116:439–46.
Sugiharto S, Ramadany S, Handayani H, Achmad H, Mutmainnah N, Inayah NH, et al. Assessment of the Anti-inflammatory Activities of the Moringa Leaf Extract in Periodontitis Cases through IL-6 Cytokine Analysis in Wistar (Rattus novergicus). Open Access Macedonian J Med Sci. 2022;10:124–30.
Jeong HM, Han EH, Jin YH, Hwang YP, Kim HG, Park BH, et al. Saponins from the roots of Platycodon grandiflorum stimulate osteoblast differentiation via p38 MAPK-and ERK-dependent RUNX2 activation. Food Chem Toxicol. 2010;48:3362–8.
Guo AJ, Choi RC, Zheng KY, Chen VP, Dong TT, Wang Z-T, et al. Kaempferol as a flavonoid induces osteoblastic differentiation via estrogen receptor signaling. Chin Med. 2012;7:1–7.
Teixeira EMB, Carvalho MRB, Neves VA, Silva MA, Arantes-Pereira L. Chemical characteristics and fractionation of proteins from Moringa oleifera Lam. leaves. Food Chem. 2014;147:51–4.
Kim SY, Park JY, Lee SH, Woo J-T, Kim H, Park EK. Inhibitory effects of hydrolisable tannins on osteoclast differentiation and function through inhibition of map kinases and ap‑1/nf‑kb activations. In: 52nd Annual Meeting of the Orthopaedic Research Society, 2006. Vol. 1, p. 1572.
Syarif RD, Kusumaningsih T, Arundina I. Changes in osteoblast and osteoclast cell count after moringa oleifera leaf extract administration during orthodontic tooth movement. J Dentomaxillofacial Sci. 2020;5:98–102.
Khan MI, Siddiqui S, Barkat MA, Alhodieb FS, Ashfaq F, Barkat HA, et al. Moringa oleifera leaf extract induces osteogenic-like differentiation of human osteosarcoma SaOS2 cells. J Traditional Complementary Med. 2022;12:608–18.
Marupanthorn K, Kedpanyapong W. The Effects of Moringa Oleifera Lam. Leaves Extract on Osteogenic Differentiation of Porcine Bone Marrow Derived Mesenchymal Stem Cells. 2017.
Stohs S, Hartman M. Revisión de la Seguridad y Eficacia de la Moringa oleifera. Phytother. Res. 2015;29:796–804.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was performed after gaining the approval of the Research Ethics Committee, Faculty of Dentistry, Alexandria University. All animals’ procedures followed the National Research Council Guidelines for the care and use of laboratory animals. Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Nouran Elsadek (NE): conceptualization, investigation, methodology, and writing of the original draft. Maha Abokhadr (MA): conception and design of the study, supervision of the surgical operations, visualization, validation, and revising the article critically and approving the final version. Fatma Ramzy (FR): conception and design of the study, supervision of the surgical operations, visualization, validation, and revising the article critically and approving the final version. Hossam Mostafa (HM): data curation, formal analysis, revising the article critically, and approving the final version. Gillan El-Kimary (GE): design of the study, validation and revising the article critically and approving the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsadek, N.A., Aboukhadr, M.A., Kamel, F.R. et al. Moringa oleifera leaf extract promotes the healing of critical sized bone defects in the mandibles of rabbits. BDJ Open 10, 22 (2024). https://doi.org/10.1038/s41405-024-00201-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41405-024-00201-y