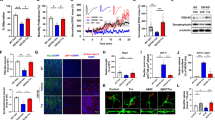
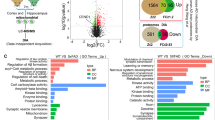
Abstract
Autophagy impairment is a key factor in Alzheimer’s disease (AD) pathogenesis. TFEB (transcription factor EB) and TFE3 (transcription factor binding to IGHM enhancer 3) are nuclear transcription factors that regulate autophagy and lysosomal biogenesis. We previously showed that corynoxine (Cory), a Chinese medicine compound, protects neurons from Parkinson’s disease (PD) by activating autophagy. In this study, we investigated the effect of Cory on AD models in vivo and in vitro. We found that Cory improved learning and memory function, increased neuronal autophagy and lysosomal biogenesis, and reduced pathogenic APP-CTFs levels in 5xFAD mice model. Cory activated TFEB/TFE3 by inhibiting AKT/mTOR signaling and stimulating lysosomal calcium release via transient receptor potential mucolipin 1 (TRPML1). Moreover, we demonstrated that TFEB/TFE3 knockdown abolished Cory-induced APP-CTFs degradation in N2aSwedAPP cells. Our findings suggest that Cory promotes TFEB/TFE3-mediated autophagy and alleviates Aβ pathology in AD models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Haque RU, Levey AI. Alzheimer’s disease: A clinical perspective and future nonhuman primate research opportunities. Proc Natl Acad Sci USA. 2019;116:26224–9.
Zhang F, Zhong RJ, Cheng C, Li S, Le WD. New therapeutics beyond amyloid-beta and tau for the treatment of Alzheimer’s disease. Acta Pharmacol Sin. 2021;42:1382–9.
Baumann KN, Sneideriene G, Sanguanini M, Schneider M, Rimon O, Gonzalez Diaz A, et al. A kinetic map of the influence of biomimetic lipid model membranes on Abeta(42) aggregation. ACS Chem Neurosci. 2022;14:323–9.
Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25:59–70.
Zhang Z, Yang X, Song YQ, Tu J. Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res Rev. 2021;72:101464.
Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci. 2015;16:345–57.
Zhang Z, Yang X, Song YQ, Tu J. Autophagy in Alzheimer’s disease pathogenesis: therapeutic potential and future perspectives. Ageing Res Rev. 2021;72:101464.
Kim JW, Jung SY, Kim Y, Heo H, Hong CH, Seo SW, et al. Identification of cathepsin D as a plasma biomarker for Alzheimer’s disease. Cells. 2021;10:138.
Zhu Z, Yang C, Iyaswamy A, Krishnamoorthi S, Sreenivasmurthy SG, Liu J, et al. Balancing mTOR signaling and autophagy in the treatment of Parkinson’s disease. Int J Mol Sci. 2019;20:728.
Hung C, Livesey FJ. Endolysosome and autophagy dysfunction in Alzheimer disease. Autophagy. 2021;17:3882–3.
Kao CH, Su TY, Huang WS, Lu XY, Jane WN, Huang CY, et al. TFEB- and TFE3-dependent autophagy activation supports cancer proliferation in the absence of centrosomes. Autophagy. 2022;18:2830–50.
Paquette M, El-Houjeiri L, C Zirden L, Puustinen P, Blanchette P, Jeong H, et al. AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy. 2021;17:3957–75.
Iyaswamy A, Wang X, Krishnamoorthi S, Kaliamoorthy V, Sreenivasmurthy SG, Kumar Durairajan SS, et al. Theranostic F-SLOH mitigates Alzheimer’s disease pathology involving TFEB and ameliorates cognitive functions in Alzheimer’s disease models. Redox Biol. 2022;51:102280.
Yang C, Zhu Z, Tong BC, Iyaswamy A, Xie WJ, Zhu Y, et al. A stress response p38 MAP kinase inhibitor SB202190 promoted TFEB/TFE3-dependent autophagy and lysosomal biogenesis independent of p38. Redox Biol. 2020;32:101445.
Kastanenka KV, Bussiere T, Shakerdge N, Qian F, Weinreb PH, Rhodes K, et al. Immunotherapy with aducanumab restores calcium homeostasis in Tg2576 mice. J Neurosci. 2016;36:12549–58.
Raben N, Puertollano R. TFEB and TFE3: linking lysosomes to cellular adaptation to stress. Annu Rev Cell Dev Biol. 2016;32:255–78.
Soriano EV, Zhang Y, Colabroy KL, Sanders JM, Settembre EC, Dorrestein PC, et al. Active-site models for complexes of quinolinate synthase with substrates and intermediates. Acta Crystallogr D Biol Crystallogr. 2013;69:1685–96.
Malek M, Wawrzyniak AM, Ebner M, Puchkov D, Haucke V. Inositol triphosphate-triggered calcium release from the endoplasmic reticulum induces lysosome biogenesis via TFEB/TFE3. J Biol Chem. 2022;298:101740.
Lee J, Jin C, Cho SY, Park SU, Jung WS, Moon SK, et al. Herbal medicine treatment for Alzheimer disease: a protocol for a systematic review and meta-analysis. Medicine. 2020;99:e21745.
Hsieh CL, Chen MF, Li TC, Li SC, Tang NY, Hsieh CT, et al. Anticonvulsant effect of Uncaria rhynchophylla (Miq) Jack. in rats with kainic acid-induced epileptic seizure. Am J Chin Med. 1999;27:257–64.
Hsieh CL, Tang NY, Chiang SY, Hsieh CT, Lin JG. Anticonvulsive and free radical scavenging actions of two herbs, Uncaria rhynchophylla (MIQ) Jack and Gastrodia elata Bl., in kainic acid-treated rats. Life Sci. 1999;65:2071–82.
Liu L, Zhao YH, Zeng CQ, Zeng Y. [Research progress in pharmacological effects of Uncaria Hook on Alzheimer disease models]. Yao Xue Xue Bao. 2016;51:536–42.
Liu LF, Song JX, Lu JH, Huang YY, Zeng Y, Chen LL, et al. Tianma Gouteng Yin, a traditional Chinese medicine decoction, exerts neuroprotective effects in animal and cellular models of Parkinson’s disease. Sci Rep. 2015;5:16862.
Zeng P, Wang XM, Ye CY, Su HF, Tian Q. The main alkaloids in Uncaria rhynchophylla and their anti-Alzheimer’s disease mechanism determined by a network pharmacology approach. Int J Mol Sci. 202;22:3612.
Chen LL, Song JX, Lu JH, Yuan ZW, Liu LF, Durairajan SS, et al. Corynoxine, a natural autophagy enhancer, promotes the clearance of alpha-synuclein via Akt/mTOR pathway. J Neuroimmune Pharmacol. 2014;9:380–7.
Tong BC, Wu AJ, Huang AS, Dong R, Malampati S, Iyaswamy A, et al. Lysosomal TPCN (two pore segment channel) inhibition ameliorates beta-amyloid pathology and mitigates memory impairment in Alzheimer’s disease. Autophagy. 2022;18:624–42.
Iyaswamy A, Krishnamoorthi SK, Song JX, Yang CB, Kaliyamoorthy V, Zhang H, et al. NeuroDefend, a novel Chinese medicine, attenuates amyloid-beta and tau pathology in experimental Alzheimer’s disease models. J Food Drug Anal. 2020;28:132–46.
Song JX, Malampati S, Zeng Y, Durairajan SSK, Yang CB, Tong BC, et al. A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and Tau pathology in Alzheimer’s disease models. Aging Cell. 2020;19:e13069.
Tong BC, Huang AS, Wu AJ, Iyaswamy A, Ho OK, Kong AH, et al. Tetrandrine ameliorates cognitive deficits and mitigates tau aggregation in cell and animal models of tauopathies. J Biomed Sci. 2022;29:85.
O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204.
Zheng X, Lin W, Jiang Y, Lu K, Wei W, Huo Q, et al. Electroacupuncture ameliorates beta-amyloid pathology and cognitive impairment in Alzheimer disease via a novel mechanism involving activation of TFEB (transcription factor EB). Autophagy. 2021;17:3833–47.
Scotto Rosato A, Montefusco S, Soldati C, Di Paola S, Capuozzo A, Monfregola J, et al. TRPML1 links lysosomal calcium to autophagosome biogenesis through the activation of the CaMKKbeta/VPS34 pathway. Nat Commun. 2019;10:5630.
Di Paola S, Scotto-Rosato A, Medina DL. TRPML1: The Ca2+ retaker of the lysosome. Cell Calcium. 2018;69:112–21.
Wang T, Wu Z, Sun L, Li W, Liu G, Tang Y. A computational systems pharmacology approach to investigate molecular mechanisms of herbal formula Tian-Ma-Gou-Teng-Yin for treatment of Alzheimer’s disease. Front Pharmacol. 2018;9:668.
Chen L, Huang Y, Yu X, Lu J, Jia W, Song J, et al. Corynoxine protects dopaminergic neurons through inducing autophagy and diminishing neuroinflammation in rotenone-induced animal models of Parkinson’s disease. Front Pharmacol. 2021;12:642900.
Song JX, Lu JH, Liu LF, Chen LL, Durairajan SS, Yue Z, et al. HMGB1 is involved in autophagy inhibition caused by SNCA/alpha-synuclein overexpression: a process modulated by the natural autophagy inducer corynoxine B. Autophagy. 2014;10:144–54.
Lu JH, Tan JQ, Durairajan SS, Liu LF, Zhang ZH, Ma L, et al. Isorhynchophylline, a natural alkaloid, promotes the degradation of alpha-synuclein in neuronal cells via inducing autophagy. Autophagy. 2012;8:98–108.
van der Welle REN, Jobling R, Burns C, Sanza P, van der Beek JA, Fasano A, et al. Neurodegenerative VPS41 variants inhibit HOPS function and mTORC1-dependent TFEB/TFE3 regulation. EMBO Mol Med. 2021;13:e13258.
Medina DL. TRPML1 and TFEB, an Intimate Affair. Handb Exp Pharmacol. 2023;278:109–26.
Acknowledgements
We would like to thank Mr. Alan Ho for provision of equipment and technical training. We would like to thank Dr. Martha Dahlen for her English editing of this manuscript. This study was supported by Hong Kong Health and Medical Research Fund (HMRF/17182551, HMRF/09203776) and the Hong Kong General Research Fund (CRF/C2011-21GF, HKBU 12101022) from Hong Kong Government. The study was partly supported by the Research Fund from Hong Kong Baptist University (HKBU/RC-IRCs/17-18/03, IRCMS/19-20/H02), GDSSIL/84-506/2019 and the National Natural Science Foundation of China (82074042).
Author information
Authors and Affiliations
Contributions
JXS, AI, and ML designed the research; XJG, AI, JL, BCKT, ZZ, CFS, YXK, and SGS and performed the main experiments; XJG, AI, KJL, and ZQD analyzed the data; XJG, ZQD, and JXS wrote the paper; XJG, ZZ, SGS, CPKC, KHC, RBP, ZQD, JXS, and ML revised the paper; JXS and ML supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guan, Xj., Deng, Zq., Liu, J. et al. Corynoxine promotes TFEB/TFE3-mediated autophagy and alleviates Aβ pathology in Alzheimer’s disease models. Acta Pharmacol Sin 45, 900–913 (2024). https://doi.org/10.1038/s41401-023-01197-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01197-1