Abstract
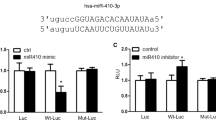
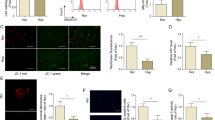
Pulmonary arterial hypertension (PH) is a chronic disease induced by a progressive increase in pulmonary vascular resistance and failure of the right heart function. A number of studies show that the development of PH is closely related to the gut microbiota, and lung-gut axis might be a potential therapeutic target in the PH treatment. A. muciniphila has been reported to play a critical role in treating cardiovascular disorders. In this study we evaluated the therapeutic effects of A. muciniphila against hypoxia-induced PH and the underlying mechanisms. Mice were pretreated with A. muciniphila suspension (2 × 108 CFU in 200 μL sterile anaerobic PBS, i.g.) every day for 3 weeks, and then exposed to hypoxia (9% O2) for another 4 weeks to induce PH. We showed that A. muciniphila pretreatment significantly facilitated the restoration of the hemodynamics and structure of the cardiopulmonary system, reversed the pathological progression of hypoxia-induced PH. Moreover, A. muciniphila pretreatment significantly modulated the gut microbiota in hypoxia-induced PH mice. miRNA sequencing analysis reveals that miR-208a-3p, a commensal gut bacteria-regulated miRNA, was markedly downregulated in lung tissues exposed to hypoxia, which was restored by A. muciniphila pretreatment. We showed that transfection with miR-208a-3p mimic reversed hypoxia-induced abnormal proliferation of human pulmonary artery smooth muscle cells (hPASMCs) via regulating the cell cycle, whereas knockdown of miR-208a-3p abolished the beneficial effects of A. muciniphila pretreatment in hypoxia-induced PH mice. We demonstrated that miR-208a-3p bound to the 3′-untranslated region of NOVA1 mRNA; the expression of NOVA1 was upregulated in lung tissues exposed to hypoxia, which was reversed by A. muciniphila pretreatment. Furthermore, silencing of NOVA1 reversed hypoxia-induced abnormal proliferation of hPASMCs through cell cycle modulation. Our results demonstrate that A. muciniphila could modulate PH through the miR-208a-3p/NOVA1 axis, providing a new theoretical basis for PH treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Deng H, Tian X, Sun H, Liu H, Lu M, Wang H. Calpain-1 mediates vascular remodelling and fibrosis via HIF-1alpha in hypoxia-induced pulmonary hypertension. J Cell Mol Med. 2022;26:2819–30.
Yan Y, Xu Y, Ni G, Wang S, Li X, Gao J, et al. MicroRNA-221 promotes proliferation and migration of pulmonary arterial smooth muscle cells (PASMCs) by targeting tissue inhibitor of metalloproteinases-3 (TIMP3). Cardiovasc Diagn Ther. 2020;10:646–57.
Yang Y, Li R, Cao Y, Dai S, Luo S, Guo Q, et al. Plasma MIR-212-3p as a biomarker for acute right heart failure with pulmonary artery hypertension. Ann Transl Med. 2020;8:1571.
Li Y, Zhang J, Sun H, Chen Y, Li W, Yu X, et al. lnc-Rps4l-encoded peptide RPS4XL regulates RPS6 phosphorylation and inhibits the proliferation of PASMCs caused by hypoxia. Mol Ther. 2021;29:1411–24.
Ma W, Qiu Z, Bai Z, Dai Y, Li C, Chen X, et al. Inhibition of microRNA-30a alleviates vascular remodeling in pulmonary arterial hypertension. Mol Ther Nucleic Acids. 2021;26:678–93.
Russomanno G, Jo KB, Abdul-Salam VB, Morgan C, Endruschat J, Schaeper U, et al. miR-150-PTPMT1-cardiolipin signaling in pulmonary arterial hypertension. Mol Ther Nucleic Acids. 2021;23:142–53.
Yi L, Liu J, Deng M, Zuo H, Li M. Emodin inhibits viability, proliferation and promotes apoptosis of hypoxic human pulmonary artery smooth muscle cells via targeting miR-244-5p/DEGS1 axis. BMC Pulm Med. 2021;21:252.
Huang L, Zhang H, Liu Y, Long Y. The role of gut and airway microbiota in pulmonary arterial hypertension. Front Microbiol. 2022;13:929752.
Kim S, Rigatto K, Gazzana MB, Knorst MM, Richards EM, Pepine CJ, et al. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020;75:1063–71.
Jia B, Zou Y, Han X, Bae JW, Jeon CO. Gut microbiome-mediated mechanisms for reducing cholesterol levels: implications for ameliorating cardiovascular disease. Trends Microbiol. 2022;31:76–91.
Hong W, Mo Q, Wang L, Peng F, Zhou Y, Zou W, et al. Changes in the gut microbiome and metabolome in a rat model of pulmonary arterial hypertension. Bioengineered. 2021;12:5173–83.
Wedgwood S, Warford C, Agvatisiri SR, Thai PN, Chiamvimonvat N, Kalanetra KM, et al. The developing gut-lung axis: postnatal growth restriction, intestinal dysbiosis, and pulmonary hypertension in a rodent model. Pediatr Res. 2020;87:472–9.
Sanada TJ, Hosomi K, Shoji H, Park J, Naito A, Ikubo Y, et al. Gut microbiota modification suppresses the development of pulmonary arterial hypertension in an SU5416/hypoxia rat model. Pulm Circ. 2020;10:2045894020929147.
Rao Y, Kuang Z, Li C, Guo S, Xu Y, Zhao D, et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes. 2021;13:1–19.
Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, et al. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front Microbiol. 2017;8:1804.
Luo Y, Zhang Y, Han X, Yuan Y, Zhou Y, Gao Y, et al. Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of TMAO induced cardiac pyroptosis. EBioMedicine. 2022;82:104087.
He X, Bai Y, Zhou H, Wu K. Akkermansia muciniphila alters gut microbiota and immune system to improve cardiovascular diseases in murine model. Front Microbiol. 2022;13:906920.
Wang G, Tao X, Peng L. miR-155-5p regulates hypoxia-induced pulmonary artery smooth muscle cell function by targeting PYGL. Bioengineered. 2022;13:12985–97.
Wang J, Jiang R, Tan Y, Cheng K. Human pulmonary artery smooth muscle cell dysfunction is regulated by miR-509-5p in hypoxic environment. Cell Cycle. 2022;21:1212–21.
Paone P, Suriano F, Jian C, Korpela K, Delzenne NM, Van Hul M, et al. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes. 2022;14:2152307.
Mody D, Verma V, Rani V. Modulating host gene expression via gut microbiome-microRNA interplay to treat human diseases. Crit Rev Microbiol. 2021;47:596–611.
Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019;11:eaav1892.
Veltman K, Hummel S, Cichon C, Sonnenborn U, Schmidt MA. Identification of specific miRNAs targeting proteins of the apical junctional complex that simulate the probiotic effect of E. coli Nissle 1917 on T84 epithelial cells. Int J Biochem Cell Biol. 2012;44:341–9.
Zhou S, Zhu C, Jin S, Cui C, Xiao L, Yang Z, et al. The intestinal microbiota influences the microenvironment of metastatic colon cancer by targeting miRNAs. FEMS Microbiol Lett. 2022;369:fnac023.
Ge XY, Zhu TT, Yao MZ, Liu H, Wu Q, Qiao J, et al. MicroRNA-137 inhibited hypoxia-induced proliferation of pulmonary artery smooth muscle cells by targeting calpain-2. Biomed Res Int. 2021;2021:2202888.
Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, et al. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. 2012;59:1006–13.
Sun L, Lin P, Chen Y, Yu H, Ren S, Wang J, et al. miR-182-3p/Myadm contribute to pulmonary artery hypertension vascular remodeling via a KLF4/p21-dependent mechanism. Theranostics. 2020;10:5581–99.
Luo L, Chen Q, Yang L, Zhang Z, Xu J, Gou D. MSCs therapy reverse the gut microbiota in hypoxia-induced pulmonary hypertension mice. Front Physiol. 2021;12:712139.
Zhao M, Wang W, Lu Y, Wang N, Kong D, Shan L. [Corrigendum] MicroRNA‑153 attenuates hypoxia‑induced excessive proliferation and migration of pulmonary arterial smooth muscle cells by targeting ROCK1 and NFATc3. Mol Med Rep. 2022;26:332.
Xing XQ, Li B, Xu SL, Liu J, Zhang CF, Yang J. MicroRNA-214-3p regulates hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration by targeting ARHGEF12. Med Sci Monit. 2019;25:5738–46.
Chen J, Zhou D, Miao J, Zhang C, Li X, Feng H, et al. Microbiome and metabolome dysbiosis of the gut-lung axis in pulmonary hypertension. Microbiol Res. 2022;265:127205.
Bachmann R, Van Hul M, Baldin P, Leonard D, Delzenne NM, Belzer C, et al. Akkermansia muciniphila reduces peritonitis and improves intestinal tissue wound healing after a colonic transmural defect by a MyD88-dependent mechanism. Cells. 2022;11:2666.
Huang CX, Jiang ZX, Du DY, Zhang ZM, Liu Y, Li YT. The MFF-SIRT1/3 axis, regulated by miR-340-5p, restores mitochondrial homeostasis of hypoxia-induced pulmonary artery smooth muscle cells. Lab Investig. 2022;102:515–23.
Oliveira AC, Yang T, Li J, Sharma RK, Karas MK, Bryant AJ, et al. Fecal matter transplant from Ace2 overexpressing mice counteracts chronic hypoxia-induced pulmonary hypertension. Pulm Circ. 2022;12:e12015.
Solinc J, Raimbault-Machado J, Dierick F, El Bernoussi L, Tu L, Thuillet R, et al. Platelet-derived growth factor receptor type alpha activation drives pulmonary vascular remodeling via progenitor cell proliferation and induces pulmonary hypertension. J Am Heart Assoc. 2022;11:e023021.
Langston-Cox A, Leo CH, Tare M, Wallace EM, Marshall SA. Sulforaphane improves vascular reactivity in mouse and human arteries after “preeclamptic-like” injury. Placenta. 2020;101:242–50.
Song K, Duan Q, Ren J, Yi J, Yu H, Che H, et al. Targeted metabolomics combined with network pharmacology to reveal the protective role of luteolin in pulmonary arterial hypertension. Food Funct. 2022;13:10695–709.
Liu C, Song C, Wang Y, Xiao Y, Zhou Z, Cao G, et al. Deep-fried Atractylodes lancea rhizome alleviates spleen deficiency diarrhea-induced short-chain fatty acid metabolic disorder in mice by remodeling the intestinal flora. J Ethnopharmacol. 2022;303:115967.
Cai H, Fan S, Cai L, Zhu L, Zhao Z, Li Y, et al. Dihydroartemisinin attenuates hypoxia-induced pulmonary hypertension through the ELAVL2/miR-503/PI3K/AKT axis. J Cardiovasc Pharmacol. 2022;80:95–109.
Luo ZW, Xia K, Liu YW, Liu JH, Rao SS, Hu XK, et al. Extracellular vesicles from Akkermansia muciniphila elicit antitumor immunity against prostate cancer via modulation of CD8+ T cells and macrophages. Int J Nanomed. 2021;16:2949–63.
Zhu L, Lu X, Liu L, Voglmeir J, Zhong X, Yu Q. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium. Vet Res. 2020;51:34.
Liu MN, Zhang L, Dong XY, Liu M, Cheng G, Zhang XL, et al. [Effects of Akkermansia muciniphila on the proliferation, apoptosis and insulin secretion of rat islet cell tumor cells]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2020;51:13–7.
Jose A, King CS, Shlobin OA, Kiernan JM, Cossa NA, Brown AW, et al. Ventricular diastolic pressure ratio as a marker of treatment response in pulmonary hypertension. Chest. 2017;152:980–9.
Pei T, Hu R, Wang F, Yang S, Feng H, Li Q, et al. Akkermansia muciniphila ameliorates chronic kidney disease interstitial fibrosis via the gut-renal axis. Micro Pathog. 2022;174:105891.
Cruz CS, Ricci MF, Vieira AT. Gut microbiota modulation as a potential target for the treatment of lung infections. Front Pharmacol. 2021;12:724033.
Xu C, Fan L, Lin Y, Shen W, Qi Y, Zhang Y, et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes. 2021;13:1980347.
Mahbobi R, Fallah F, Behmanesh A, Yadegar A, Hakemi-Vala M, Ehsanzadeh SJ, et al. Helicobacter pylori infection mediates inflammation and tumorigenesis-associated genes through miR-155-5p: an integrative omics and bioinformatics-based investigation. Curr Microbiol. 2022;79:192.
Fu Y, Wang Y, Bi K, Yang L, Sun Y, Li B, et al. MicroRNA-208a-3p promotes osteosarcoma progression via targeting PTEN. Exp Ther Med. 2020;20:255.
Zhang Y, Li L, Liang P, Zhai X, Li Y, Zhou Y. Differential expression of microRNAs in medulloblastoma and the potential functional consequences. Turk Neurosurg. 2018;28:179–85.
Wu H, Xu L, Chen Y, Xu C. MiR-208a-3p functions as an oncogene in colorectal cancer by targeting PDCD4. Biosci Rep. 2019;39:BSR20181598.
Krach F, Wheeler EC, Regensburger M, Boerstler T, Wend H, Vu AQ, et al. Aberrant NOVA1 function disrupts alternative splicing in early stages of amyotrophic lateral sclerosis. Acta Neuropathol. 2022;144:413–35.
Brahma MK, Xiao P, Popa M, Negueruela J, Vandenbempt V, Demine S, et al. Nova1 or Bim deficiency in pancreatic beta-cells does not alter multiple low-dose streptozotocin-induced diabetes and diet-induced obesity in mice. Nutrients. 2022;14:3866.
Wang ZY, Liu XX, Deng YF. Negative feedback of SNRK to circ-SNRK regulates cardiac function post-myocardial infarction. Cell Death Differ. 2022;29:709–21.
Pedraza-Arevalo S, Alors-Perez E, Blazquez-Encinas R, Herrera-Martinez AD, Jimenez-Vacas JM, Fuentes-Fayos AC, et al. Spliceosomic dysregulation unveils NOVA1 as a candidate actionable therapeutic target in pancreatic neuroendocrine tumors. Transl Res. 2022;251:63–73.
Sun S, Wang R, Yi S, Li S, Wang L, Wang J. Roles of the microRNA-338-3p/NOVA1 axis in retinoblastoma. Mol Med Rep. 2021;23:394.
Ludlow AT, Wong MS, Robin JD, Batten K, Yuan L, Lai TP, et al. NOVA1 regulates hTERT splicing and cell growth in non-small cell lung cancer. Nat Commun. 2018;9:3112.
Acknowledgements
The research was supported by the National Key Research and Development Program of China (2021YFA1301100, 2021YFA1301101, 2022YFA1303801), the Research Project of Ji-nan Microecological Biomedicine Shandong Laboratory (JNL-2022001A) and the Fundamental Research Funds for the Central Universities (No. 2022ZFJH003).
Author information
Authors and Affiliations
Contributions
LJL and MFY designed the research and wrote the paper. ZYB and HML performed major experiments and analyzed the data. SBZ and YQF performed animal experiments. All the authors approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bao, Zy., Li, Hm., Zhang, Sb. et al. Administration of A. muciniphila ameliorates pulmonary arterial hypertension by targeting miR-208a-3p/NOVA1 axis. Acta Pharmacol Sin 44, 2201–2215 (2023). https://doi.org/10.1038/s41401-023-01126-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01126-2