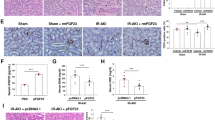
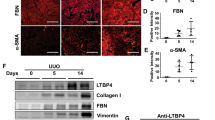
Abstract
Damage to peritubular capillaries is a key process that contributes to acute kidney injury (AKI) progression. Vascular endothelial growth factor A (VEGFA) plays a critical role in maintaining the renal microvasculature. However, the physiological role of VEGFA in various AKI durations remains unclear. A severe unilateral ischemia‒reperfusion injury model was established to provide an overview of VEGFA expression and the peritubular microvascular density from acute to chronic injury in mouse kidneys. Therapeutic strategies involving early VEGFA supplementation protecting against acute injury and late anti-VEGFA treatment for fibrosis alleviation were analyzed. A proteomic analysis was conducted to determine the potential mechanism of renal fibrosis alleviation by anti-VEGFA. The results showed that two peaks of extraglomerular VEGFA expression were observed during AKI progression: one occurred at the early phase of AKI, and the other occurred during the transition to chronic kidney disease (CKD). Capillary rarefaction progressed despite the high expression of VEGFA at the CKD stage, and VEGFA was associated with interstitial fibrosis. Early VEGFA supplementation protected against renal injury by preserving microvessel structures and counteracting secondary tubular hypoxic insults, whereas late anti-VEGFA treatment attenuated renal fibrosis progression. The proteomic analysis highlighted an array of biological processes related to fibrosis alleviation by anti-VEGFA, which included regulation of supramolecular fiber organization, cell-matrix adhesion, fibroblast migration, and vasculogenesis. These findings establish the landscape of VEGFA expression and its dual roles during AKI progression, which provides the possibility for the orderly regulation of VEGFA to alleviate early acute injury and late fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–66.
Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26:1765–76.
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12:7–11.
Mantovani A, Zusi C. PNPLA3 gene and kidney disease. Exploration Med. 2020;1:42–50.
Rashid I, Katravath P, Tiwari P, D’Cruz S, Jaswal S, Sahu G. Hyperuricemia—a serious complication among patients with chronic kidney disease: a systematic review and meta-analysis. Exploration Med. 2022;3:249–59.
Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–26.
Tanabe K, Wada J, Sato Y. Targeting angiogenesis and lymphangiogenesis in kidney disease. Nat Rev Nephrol. 2020;16:289–303.
Tanaka S, Tanaka T, Nangaku M. Hypoxia and dysregulated angiogenesis in kidney disease. Kidney Dis. 2015;1:80–9.
Mao H, Jiang C, Xu L, Chen D, Liu H, Xu Y, et al. Ginsenoside protects against AKI via activation of HIF‑1α and VEGF‑A in the kidney‑brain axis. Int J Mol Med. 2020;45:939–46.
Liu F, Lou YL, Wu J, Ruan QF, Xie A, Guo F, et al. Upregulation of microRNA-210 regulates renal angiogenesis mediated by activation of VEGF signaling pathway under ischemia/perfusion injury in vivo and in vitro. Kidney Blood Press Res. 2012;35:182–91.
Villanueva S, Cespedes C, Gonzalez A, Vio CP. bFGF induces an earlier expression of nephrogenic proteins after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1677–87.
Qin LL, Xue F, Yin F, Zhao J, Zhang KY. Expression of syndecan-1, PKC and VEGF in rats with acute kidney injury and correlation between syndecan-1 and renal function. Eur Rev Med Pharmacol Sci. 2020;24:12794–801.
Sánchez-Navarro A, Pérez-Villalva R, Murillo-de-Ozores AR, Martínez-Rojas M, Rodríguez-Aguilera JR, González N, et al. Vegfa promoter gene hypermethylation at HIF1α binding site is an early contributor to CKD progression after renal ischemia. Sci Rep. 2021;11:8769.
Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Ren Physiol. 2008;294:F928–36.
Tögel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med. 2009;13:2109–14.
Bai Y, Zhang Y, Yang S, Wu M, Fang Y, Feng J, et al. Protective effect of vascular endothelial growth factor against cardiopulmonary bypass-associated acute kidney injury in beagles. Exp Ther Med. 2018;15:963–9.
Xu Y, Jiang W, Zhong L, Li H, Bai L, Chen X, et al. miR-195-5p alleviates acute kidney injury through repression of inflammation and oxidative stress by targeting vascular endothelial growth factor A. Aging. 2020;12:10235–45.
Sahin H, Borkham-Kamphorst E, Kuppe C, Zaldivar MM, Grouls C, Al-samman M, et al. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology. 2012;55:1610–9.
Zhao H, Bian H, Bu X, Zhang S, Zhang P, Yu J, et al. Targeting of discoidin domain receptor 2 (DDR2) prevents myofibroblast activation and neovessel formation during pulmonary fibrosis. Mol Ther. 2016;24:1734–44.
Barratt SL, Blythe T, Ourradi K, Jarrett C, Welsh GI, Bates DO, et al. Effects of hypoxia and hyperoxia on the differential expression of VEGF-A isoforms and receptors in Idiopathic Pulmonary Fibrosis (IPF). Respir Res. 2018;19:9.
Maurer B, Distler A, Suliman YA, Gay RE, Michel BA, Gay S, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis. 2014;73:1880–7.
Lin Y, Dong MQ, Liu ZM, Xu M, Huang ZH, Liu HJ, et al. A strategy of vascular-targeted therapy for liver fibrosis. Hepatology. 2022;76:660–75.
Lee KS, Park SJ, Kim SR, Min KH, Lee KY, Choe YH, et al. Inhibition of VEGF blocks TGF-beta1 production through a PI3K/Akt signalling pathway. Eur Respir J. 2008;31:523–31.
Lian YG, Zhou QG, Zhang YJ, Zheng FL. VEGF ameliorates tubulointerstitial fibrosis in unilateral ureteral obstruction mice via inhibition of epithelial-mesenchymal transition. Acta Pharmacol Sin. 2011;32:1513–21.
Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–57.
Chiba T, Peasley KD, Cargill KR, Maringer KV, Bharathi SS, Mukherjee E, et al. Sirtuin 5 regulates proximal tubule fatty acid oxidation to protect against AKI. J Am Soc Nephrol. 2019;30:2384–98.
Chen YT, Jhao PY, Hung CT, Wu YF, Lin SJ, Chiang WC, et al. Endoplasmic reticulum protein TXNDC5 promotes renal fibrosis by enforcing TGFβ signaling in kidney fibroblasts. J Clin Invest. 2021;131:e143645.
Zhu H, Liao J, Zhou X, Hong X, Song D, Hou FF, et al. Tenascin-C promotes acute kidney injury to chronic kidney disease progression by impairing tubular integrity via αvβ6 integrin signaling. Kidney Int. 2020;97:1017–31.
Liu D, Lun L, Huang Q, Ning Y, Zhang Y, Wang L, et al. Youthful systemic milieu alleviates renal ischemia-reperfusion injury in elderly mice. Kidney Int. 2018;94:268–79.
Chen JW, Huang MJ, Chen XN, Wu LL, Li QG, Hong Q, et al. Transient upregulation of EGR1 signaling enhances kidney repair by activating SOX9(+) renal tubular cells. Theranostics. 2022;12:5434–50.
Wang J, Sun X, Wang X, Cui S, Liu R, Liu J, et al. Grb2 induces cardiorenal syndrome type 3: roles of IL-6, cardiomyocyte bioenergetics, and Akt/mTOR pathway. Front Cell Dev Biol. 2021;9:630412.
Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight. 2017;2:e94716.
Zhang M, Wu L, Deng Y, Peng F, Wang T, Zhao Y, et al. Single cell dissection of epithelial-immune cellular interplay in acute kidney injury microenvironment. Front Immunol. 2022;13:857025.
Mohandas R, Dass B, Ejaz AA. Kinetics of vascular endothelial growth factor and endothelin 1 levels in acute kidney injury. Am J Kidney Dis. 2019;74:712–3.
Bouchard J, Mehta RL. Angiogenesis markers and recovery from acute kidney injury: a piece of the puzzle? Am J Kidney Dis. 2019;74:12–14.
Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–47.
Maciel TT, Coutinho EL, Soares D, Achar E, Schor N, Bellini MH. Endostatin, an antiangiogenic protein, is expressed in the unilateral ureteral obstruction mice model. J Nephrol. 2008;21:753–60.
Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, et al. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Ren Physiol. 2011;300:F721–33.
Lovisa S, Fletcher-Sananikone E, Sugimoto H, Hensel J, Lahiri S, Hertig A, et al. Endothelial-to-mesenchymal transition compromises vascular integrity to induce Myc-mediated metabolic reprogramming in kidney fibrosis. Sci Signal. 2020;13:eaaz2597.
LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–53.
Agarwal R, Duffin KL, Laska DA, Voelker JR, Breyer MD, Mitchell PG. A prospective study of multiple protein biomarkers to predict progression in diabetic chronic kidney disease. Nephrol Dial Transpl. 2014;29:2293–302.
Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–17.
Engel JE, Williams ML, Williams E, Azar C, Taylor EB, Bidwell GL, et al. Recovery of renal function following kidney-specific VEGF therapy in experimental renovascular disease. Am J Nephrol. 2020;51:891–902.
Qin Z, Li X, Yang J, Cao P, Qin C, Xue J, et al. VEGF and Ang-1 promotes endothelial progenitor cells homing in the rat model of renal ischemia and reperfusion injury. Int J Clin Exp Pathol. 2017;10:11896–908.
Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Ren Physiol. 2008;295:F1648–57.
Mansour SG, Zhang WR, Moledina DG, Coca SG, Jia Y, Thiessen-Philbrook H, et al. The association of angiogenesis markers with acute kidney injury and mortality after cardiac surgery. Am J Kidney Dis. 2019;74:36–46.
Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol. 2019;30:187–200.
Acknowledgements
This work was supported by Beijing Natural Science Foundation (7222169); National Natural Science Foundation of China (82000631, 82070741, 82030025, 82100713 and 32200579); Young Elite Scientist Sponsorship Program by CAST (YESS20200400); National Key Research and Development Project (2018YFE0126600); Young Talent Project of Chinese PLA General Hospital (20230404).
Author information
Authors and Affiliations
Contributions
MJH: experiments design, collection and assembly of data, data analysis and interpretation, and manuscript writing. YWJ: animal surgery, collection and assembly of data, data analysis and interpretation. JWC and LLW: data analysis and interpretation. DL, TZ, XFL, YFZ, and XY: conducting experiments. PQ and XW: performing all bioinformatics associated with this study. XFS, GYC and XMC: experiments design. ZF and QH: financial support, conception and design, manuscript revision, final approval of manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Mj., Ji, Yw., Chen, Jw. et al. Targeted VEGFA therapy in regulating early acute kidney injury and late fibrosis. Acta Pharmacol Sin 44, 1815–1825 (2023). https://doi.org/10.1038/s41401-023-01070-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01070-1