Abstract
Early life stress (ELS) significantly increases susceptibility to alcohol use disorder (AUD) by affecting the interplay between the executive and the salience networks (SNs). The link between AUD and higher body-mass index (BMI) is known, but we lack understanding of how BMI impacts the relationship between ELS and brain connectivity in individuals with AUD. To bridge this gap, we investigated the main and interaction effects of ELS and BMI on brain connectivity in individuals with AUD compared to non-AUD participants (n = 77 sex-matched individuals per group). All participants underwent resting-state functional magnetic resonance imaging, revealing intriguing positive functional connectivity between SN seeds and brain regions involved in somatosensory processing, motor coordination and executive control. Examining the relationship of brain connectivity with ELS and BMI, we observed positive associations with the correlations of SN seeds, right anterior insula (RAIns) and supramarginal gyrus (SMG) with clusters in motor [occipital cortex, supplementary motor cortex]; anterior cingulate cortex (ACC) with clusters in frontal, or executive, control regions (middle frontal gyrus; MFG, precentral gyrus) that reportedly are involved in processing of emotionally salient stimuli (all |β | > 0.001, |p | < 0.05). Interestingly, a negative association of the interaction effect of ELS events and BMI measures with the functional connectivity of SN seeds ACC with decision-making (MFG, precentral gyrus), RAIns and RSMG with visuo-motor control regions (occipital cortex and supplementary motor cortex) (all |β | = −0.001, |p | < 0.05). These findings emphasize the moderating effect of BMI on ELS-associated SN seed brain connectivity in AUD. Understanding the neural mechanisms linking BMI, ELS and AUD can guide targeted interventions for this population.
Similar content being viewed by others
Introduction
Early life stress (ELS), as determined by self-reported traumatic childhood events, such as emotional abuse, severe family conflict, domestic violence and bullying, has been linked to increased vulnerability to the development of alcohol use disorder (AUD) [1,2,3]. Individuals with AUD and obesity exhibit poor decision-making abilities, which has been linked to disruptions in the salience network (SN) assessed in resting state functional MRI studies [4, 5]. This maladaptive decision-making has been attributed to difficulties in switching between executive and salience networks in alcohol drinkers [6]. Furthermore, a decrease in resting state functional connectivity within the default mode network (DMN) and an increase in connectivity between the reward, limbic and SNs have been reported in obesity [7]. Accordingly, studies have extensively examined the relationship between heavy alcohol consumption and increased body weight over the past years [8].
It has been speculated that heavy alcohol consumption can lead to a higher body-mass index (BMI) via insulin resistance [9, 10]. Although the evidence for a comorbidity between obesity and AUD is conflicting [11], the clinical relationship between these two disorders is complex and both are affected by common aspects of vulnerability to adverse events, as well as subsequent events whereby excess alcohol consumption can lead to both weight gain and to weight loss [8, 12, 13]. Previous studies have shown that ELS is associated with alterations in brain structure and function, including reduced centrality (defined as the impact of a particular region of the brain on the transmission and exchange of information within extensive networks of the brain) in SN regions, such as the anterior insula (AIns) and dorsal anterior cingulate cortex (ACC) [14,15,16].
Despite extensive research conducted on the topic, there is still insufficient evidence of any association of ELS-related events and BMI on alterations of brain connectivity of salience, executive, somatosensory and impulse control networks in individuals with AUD. In our earlier study using data from the human connectome project (HCP) we found notable associations between chronic alcohol consumption, BMI and the decision-making capabilities for monetary rewards in people with high-risk AUD and obesity symptoms [17]. Expanding on these findings, our present aim is to investigate the similarities in the association between BMI and ELS and the resting-state seed-based functional connectivity of decision-making related regions in individuals diagnosed with AUD compared to those without an AUD diagnosis. We hypothesize that in individuals with AUD compared to those without AUD, early life stress and BMI may exhibit a positive association with the connectivity of the salience network node to brain regions involved in executive control and decision-making processes.
Materials and methods
Participants
The study cohort, as presented in Table 1, was comprised of 154 sex-matched participants categorized into those with (n = 77) and without (n = 77) a diagnosis of AUD. All participants underwent resting state functional MRI scans. AUD participants in this study were treatment-seekers and were inpatients admitted in the NIAAA clinic for treatment and their resting state functional MRI scans were obtained following detoxification and withdrawal period from alcohol. Diagnosis of AUD was made via the Structured Clinical Interview for the Diagnostic Statistical Manual (DSM)-IV or DSM-5 (SCID) [18,19,20]. For the current analysis, individuals who were diagnosed with alcohol dependence or abuse via SCID-IV were considered to have an AUD. Daily alcohol consumption in the 90 days preceding the study was assessed using the timeline follow-back (TLFB) method [21]. Individuals with substance use disorders other than AUD and nicotine dependence were excluded. Patients were allowed to smoke during their stay but were requested not to smoke or remove their nicotine patch two hours prior to their MRI scan. The study was approved by the Institutional Review Board of the National Institutes of Health, and all participants provided written informed consent to participate.
Body-mass index (BMI)
BMI for all participants in the study was calculated by dividing their body weight by the square of their height (Kg/m2). Measurements of both body weight and height were recorded when participants enrolled in the NIAAA natural history study. The continuous BMI variable was utilized to conduct further analysis to identify the relationship between BMI, and ELS on brain connectivity patterns in AUD vs. non-AUD participants.
Early life stress (ELS) events
ELS was operationalized using self-report questionnaire which consists of 19 standard life event items experienced in their early life as a child (up to age 18 years). The participants’ responses were recorded as yes/no for each life event including emotional, sexual, and physical abuse, as well as violence, negligence, parental divorce, surgery, parental death, separation and so forth [22, 23]. The sum of responses for all domains was used to create a complete ELS_events score (with a maximum score of 19; Mean = 3.58, SD = 3.2). Emotional abuse, severe family conflict, domestic violence and bullying were the most reported events.
Resting-state functional MRI (rsfMRI) data acquisition and preprocessing
Resting-state fMRI (rs-fMRI) scans were acquired from patients during the inpatient treatment phase when they were stabilized and not experiencing stress or acute withdrawal symptoms. Patients’ withdrawal scores were assessed using the Clinical Institute Withdrawal Assessment for Alcohol-revised (CIWA-Ar), a 10-item scale [24]. To be eligible for rs-fMRI scans, patients had to have an average CIWA-Ar score below 8, which typically occurs at 1 week after admission, and the scans were done during weeks 2 or 3 of their inpatient stay. The scans were conducted at the NIH NMR Center, utilizing a Siemens 3 T MRI Skyra scanner with a 20-channel head coil. Participants were instructed to keep their eyes open and remain alert during the ten-minute period of rs-fMRI data collection, with no additional stimuli presented. The rs-fMRI scans were acquired utilizing an echoplanar-imaging pulse sequence (TR: 2000 ms, TE: 30 ms, FA: 90°, FOV: 240 × 240 mm, 3.8 mm slice thickness, multi-slice mode: interleaved). A high-resolution T1-weighted MPRAGE (TR: 1900 ms, TE: 3.09 ms, FA: 10°, FOV: 240 × 240 mm, 1 mm slice thickness) was obtained for registration purposes. Preprocessing of the data was carried out using the CONN toolbox (version 18.b; https://www.nitrc.org/executedashboard/?group_id=279), a Matlab-based toolbox for functional connectivity analysis (http://www.nitrc.org/projects/conn) [25], including realignment and unwarp, slice-timing correction, outlier identification, and normalization. Artifact detection was performed based on scan-to-scan differences in the global signal (z-value threshold 5) and subject motion parameters (threshold 0.9 mm) using the ‘art’ software for artifact rejection (www.nitrc.org/projects/artifact_detect/), with identified outlier scans included as first-level covariates.
Functional connectivity
To analyze rs-fMRI data, we used the CONN toolbox (18.b) with full width at half maximum spatial smoothing of 8 mm. To minimize effects of head motion, we regressed out principal components associated with segmented white matter and cerebrospinal fluid using CompCor [26], as well as twelve motion regressors (3 rotational, 3 translational, and their derivatives) calculated from CONN image preprocessing. The data were filtered using a band-pass filter of 0.008-0.09 Hz to eliminate very-low-frequency drift and high frequency noise, and linear trends were removed. We used a continuous squashing function (i.e., despiking) to further minimize the influence of potential outlier scans. Global BOLD signal was not regressed out to avoid the mathematical introduction of negative correlations [27].
We conducted a seed to voxel (whole brain) resting state connectivity analysis to investigate the influence of ELS on the connectivity of SN seed regions and the rest of the brain. The seeds were selected a priori based on our hypothesis of strong interaction of SN with executive function networks in addictive conditions [28,29,30]. The seeds were defined based on the anatomical FSL Harvard-Oxford atlas, which is the default atlas utilized for segmentation during the CONN processing procedure. We included the anterior insula, anterior cingulate cortex, and inferior parietal cortex (supramarginal gyrus) as seed regions associated with the SN. A separate model was created for the left and right structures for each seed. We extracted the mean time series of the seed region from their preprocessed functional data and calculated Pearson’s correlation coefficients for the connection between the seed and voxel for each participant. To enable further analyses, we transformed the resulting values into normally distributed Z-scores using the Fisher transformation. The identified correlations are presented in the results section.
Statistical analyses
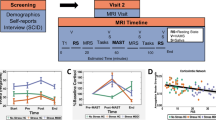
To compare the demographic and clinical characteristics of our study groups (AUD vs. non-AUD), we utilized Student’s t-tests and Mann–Whitney tests for continuous variables, and chi-squared tests for categorical variables. The age and sex-controlled connectivity coefficients between the SN seeds and significant clusters in the AUD compared to non-AUD group were extracted from CONN. We considered connection-level False Discovery Rate (FDR)-corrected P values < 0.05 as significant [31]. We then employed general linear model (GLM) to investigate the relationship of ELS events and BMI and the interaction effect of ELS events and BMI measures with the correlations of seed and significant clusters (interpreted as connectivity, the dependent variable) and included ELS events, BMI, age, sex, smoking status, and AUD status as fixed factors. For all descriptives and regression models, we used SPSS 22 (IBM Corp., Armonk, NY). Figure 1 provides an overview of the study flow, including participant measures and statistical approach used to investigate the study hypothesis.
The alcohol use disorder (AUD) and non-AUD population. The measures included early life stress (ELS) events, body-mass-index (BMI) and resting-state functional connectivity (rs-fMRI). The main analyses consisted of seed-based rs-fMRI to identify connectivity patterns in individuals with AUD, as well as General Linear Models to identify relationships of connectivity differences with ELS events and BMI as well as the interaction effects of ELS events and BMI measures.
Results
Characteristics of the sample: demographic and clinical
There was a significant difference in age distribution between the AUD and non-AUD cohorts (test statistic = −5.6; p < 0.001). The full BMI and ELS distribution of the AUD and non-AUD cohorts are reported in Supplementary Fig. S1. The data show a significant difference in distribution of ELS across the AUD vs. non-AUD cohorts (test statistic = −5.5; p < 0.001), while BMI distribution was not significantly different across the study cohorts (test statistic = 0.18; p = 0.86).
Furthermore, the percentage of smokers was significantly higher in the AUD cohort (64.9%) compared to the non-AUD cohort (1.3%; p < 0.001). Individuals with AUD had a lower level of education (mean years of education, 13.5 ± 2.7) and had low household income (30K-39, 999) compared to participants without AUD [(mean years of education, 16.5 ± 3.7; p < 0.001) (household income, 50K-74, 999; p = 0.002)]. No significant difference in race and ethnicity was identified between the AUD and non-AUD groups (refer to Table 1).
Resting state fMRI
Seed-based voxel connectivity (SBVC) in AUD compared to non-AUD
Several clusters displayed altered connectivity with our seed regions, including the left/right SMG, left/right AIns, and ACC, following adjustment for age and sex in the AUD compared to the non-AUD cohort. For further information on the connected clusters, please refer to Table 2. Additionally, a visual representation of the extent of significance can be found in Fig. 2.
A–D Significant Seed-to-Voxel connections representing salience network seed regions (anterior cingulate cortex, anterior insula left/right and supramarginal gyrus left/right). Labels provided for perspective reference are as follows: SMC Supplementary Motor Cortex, MFG Middle Frontal Gyrus, PoG Postcentral Gyrus, LOC Lateral Occipital Cortex, SPL Superior Parietal Lobule; cluster of significant activation at the peak-wise PFWE < 0.001/cluster size P < 0.05 FDR corrected level. Directions of connectivity are noted in Table 2.
The ACC seed region demonstrated positive connectivity with clusters in both the right and left frontal pole, postcentral gyrus, precentral gyrus, and middle frontal gyrus (MFG), as well as the left lateral occipital cortex (LOC), superior parietal lobule (SPL), and caudate. Additionally, it exhibited connections with the right thalamus, posterior supramarginal gyrus, and precuneus cortex. The left AIns seed exhibited positive connectivity with a cluster in the left postcentral gyrus. Conversely, the right AIns seed displayed positive connections with the right frontal pole, supplementary motor cortex, precentral gyrus, and postcentral gyrus (see Table 2; Fig. 2A–C).
The left SMG seed exhibited positive connectivity with various clusters, including the right LOC, postcentral gyrus, precentral gyrus, and frontal pole. In contrast, the right SMG seed demonstrated negative connectivity with the left precentral gyrus, while displaying positive connectivity with the right LOC, anterior supramarginal gyrus, postcentral gyrus, and SPL (refer to Table 2; Fig. 2D, E).
Association of ELS events and BMI with SBVC in individuals with AUD versus non-AUD
Following adjustment for age, sex, smoking status, and AUD status in our GLM analysis, we identified several associations of SBVC in the AUD versus non-AUD cohort with both ELS events and BMI. Specifically, we observed positive associations between increased occurrences of ELS events and BMI measure with the connectivity of the ACC with left MFG [ELS events: (β = 0.03; CI = 0.01, 0.01; p = 0.01), BMI: (β = 0.01; CI = 0.001, 0.01; p = 0.01)] and bilateral precentral gyrus [ELS: left, (β = 0.02; CI = 0.002, 0.04; p = 0.03), right, (β = 0.03; CI = 0.004, 0.05; p = 0.02); BMI: left, (β = 0.004; CI = 2.3E–5, 0.006; p = 0.04), right precentral gyrus (β = 0.01; CI = 0.003, 0.01; p = 0.002)] clusters. Functional connectivity of RAIns seed with right supplementary motor cortex cluster was also significantly positively correlated with both ELS events (β = 0.02; CI = 0.01, 0.03; p = 0.006) and BMI measures (β = 0.001; CI = 0.002, 0.01; p = 0.03). Functional connectivity of RSMG seed with right LOC cluster was also significantly positively correlated with both ELS events (β = 0.02; CI = 0.003, 0.04; p = 0.02) and BMI measures (β = 0.01; CI = 0.001, 0.01; p = 0.009).
We also identified an interaction effect of ELS events and BMI measures on these connectivity patterns in AUD vs. non-AUD cohort. ELS events*BMI was negatively associated with the connectivity patterns: ACC seed connection with left MFG (β = −0.001; CI = −0.002, 7.67E–5; p = 0.03) and bilateral precentral gyrus [left, (β = −0.001; CI = (−0.001, 6.09E–5; p = 0.04); right, (β = −0.001; CI = 0.002, 0.00; p = 0.02)]); RAIns seed connection with right supplementary motor cortex (β = −0.001; CI = 0.001, 0.00; p = 0.01); RSMG seed connection with right LOC (β = −0.001; CI = 0.002, −2.75E–5; p = 0.04) (see Table 2 for details).
Discussion
The current study sought to investigate the functional connectivity of SN seed in AUD versus non-AUD participants and their association with history of ELS events and BMI measures. As predicted, SBVC in AUD versus non-AUD study participants revealed several positive connectivity patterns of SN seeds, including ACC, bilateral AIns and bilateral SMG with whole brain clusters in somatosensory and motor coordination areas (such as the bilateral LOC, supplementary motor cortex, postcentral gyrus, and supramarginal gyrus); frontal, or executive control regions (e.g., key nodes of the fronto-parietal network: MFG, precentral gyrus, SPL); and nodes in posterior DMN (precuneus, thalamus and caudate).
The connectivity patterns identified in participants with AUD (vs. non-AUD) in our study is in alignment with a previous report in participants with AUD wherein increased within and between SN, DMN and executive control networks functional connectivity were noted in AUD compared to healthy controls using a whole-brain probabilistic independent component analysis approach [32]. This heightened functional connectivity between the SN seed regions, specifically the insula and anterior cingulate cortex (ACC) and the visual cortex (LOC) and middle frontal gyrus (MFG), was also detected in a study by Han et al. at a moderate alcohol dose [33]. The observed increased connectivity between the SN seed regions and somatosensory and motor coordination areas may be attributed to an enhanced involvement of the SN in detecting and assigning emotional significance to relevant sensory stimuli. This interpretation finds further support in reports of increased connectivity between the ACC and the sensorimotor network [34] and is consistent with the visuomotor effects associated with alcohol [35, 36]. In line with previous reports suggesting a crucial role of DMN nodes, such as precuneus in social and self-related cognitive processes [37, 38], the increased ACC-precuneus coupling identified in our study might pertain to heightened self-awareness and emotional response to negative social stimuli in AUD subjects. This, in turn, could potentially increase impulsive decision-making and drinking behaviors as a way of regulating these emotions in individuals with AUD (vs. non-AUD). Additionally, as previously highlighted in our own studies [32, 39], the heightened functional connectivity identified in individuals with AUD compared to those without AUD may indicate a potential neural mechanism of compensation or adaptation following long-term alcohol exposure, wherein the structural damage resulting from chronic alcohol use [40,41,42] is potentially restored through the dynamic coupling of related networks, including the SN, motor coordination networks and DMN.
In our population which comprised of moderate-to-severe AUD and non-AUD subjects, BMI distribution did not reveal a significant difference; however, a clear difference in the distribution of ELS events between patients with AUD and without AUD was seen. This hints that the relationship of BMI and ELS in the context of AUD may not be straightforward, and there may be other factors that have a stronger impact on the association between ELS and BMI in individuals with AUD. Consequently, upon examining the association of history of ELS events and BMI with the identified connectivity in AUD (vs. non-AUD) subjects, we identified several clusters that were associated with both ELS and BMI increases.
Notably both ELS events and BMI were positively associated with the functional connectivity between SN seeds, RAIns and RSMG with clusters in motor [occipital cortex, supplementary motor cortex], ACC with clusters in frontal, or executive, control regions (MFG, precentral gyrus) that reportedly are involved in processing of emotionally salient stimuli [43,44,45,46]. Exposure to stress during critical periods of brain development has been demonstrated to modify connectivity patterns and heighten the risk of developing AUD. Likewise, numerous studies provide evidence that experiencing ELS has harmful effects on individuals and enhances their susceptibility to alcohol use in adulthood [2, 47,48,49,50,51]. Moreover, exposure to a series of ELS events leads to modifications in connectivity of brain regions associated with emotion, self-regulation and cognition, including nodes within the fronto-limbic networks, such as the mPFC, ACC, amygdala and orbitofrontal cortex [52,53,54]. Children between the ages of 9 and 16 who were exposed to various stress events, such as conventional crimes, child maltreatment, peer/sibling victimization and sexual victimization, were found to have a reduced functional connection between their SMG and PCC [55]. Moreover, it is noteworthy that exposure to acute stress has been linked with elevated functional connectivity between the nodes of the default mode and SNs in healthy adults and adolescents [55, 56]. Considering the diverse range of ELS events encountered by the participants in our study, spanning from a single event to as many as nineteen stressors, it is conceivable that the directional connectivity between the SN and various brain regions was influenced by the cumulative extent of their early life stress experiences. Moreover, these connectivity patterns exhibited a positive association with the elevation of BMI, suggesting heightened functional connectivity between the SN and brain areas implicated in decision-making processes and the coordination of visual and motor functions in individuals with AUD. The findings suggest two potential scenarios: either ELS contributes to overeating in individuals with AUD, or ELS-induced overeating could heighten their vulnerability to excessive alcohol consumption. Consequently, the connections of the SN with regions governing decision-making (fronto-parietal) and the coordination of visual and motor functions (lateral occipital cortex and supplementary motor cortex) might encounter further impact in individuals with AUD due to the co-occurring effects of early life stress and heightened BMI, thereby leading to irregular processing of salient stimuli. To test this hypothesis, we assessed the interaction effect of assessment of the interaction effect between the frequency of ELS events and BMI measurements with the identified patterns of functional connectivity in individuals with AUD as compared to those without AUD.
Interestingly, we identified a negative association of the interaction effect of ELS events and BMI measures with the functional connectivity of SN seeds ACC with decision-making (MFG, precentral gyrus), RAIns and RSMG with visuo-motor control regions (LOC and supplementary motor cortex). Both pre-clinical and clinical studies have identified the contribution of ELS in increasing the risk for obesity [57,58,59] and AUD [48, 60, 61], which was attributed to persistent overactivation of the hypothalamic-pituitary-adrenal (HPA) axis [62], dysregulation of the mesolimbic dopamine functions [63, 64] and an imbalance in connectivity patterns of salience, emotion and somatosensory networks [65]. Nonetheless, none of these studies demonstrated the combined relationship of increased occurrence of ELS events and BMI measures on brain connectivity in adults with AUD compared to those without AUD. Our results suggest that the increase in both ELS events and BMI disrupts the connectivity of SN with decision-making and visuo-motor coordination regions, potentially amplifying impulsive decision-making and compromising self-control behaviors. These altered behaviors, influenced by ELS and exacerbated by an increase in BMI, may be interpreted as the underlying drivers for the worsening of early life stress history related AUD [66].
The present study offers intriguing insights into the intricate relationship between ELS, BMI and AUD and the connections of salience network seeds with the whole brain. These findings corroborate our initial hypothesis, which postulates that heightened BMI might influence the connectivity patterns between the SN and specific brain regions responsible for regulating executive control and impulsive behaviors, particularly in individuals diagnosed with AUD and a history of early life stress. Moreover, the notable detrimental effect on the connectivity of SN seeds with fronto-parietal and visuo-motor coordination networks, specifically in AUD individuals (vs. non-AUD), because of increased BMI stemming from a higher frequency of stressful events during early life, implies a potential neurobiological mechanism through which the combination of elevated BMI and a history of early life stress contributes to alterations in the functional connectivity of crucial brain networks in these individuals. Further exploration of these intricate associations can significantly enhance our understanding of the neurobiological underpinnings of AUD, especially in the context of early life adversity and its impact on BMI.
Limitations
There are several unanswered questions that need to be explored in future large cohort studies. One limitation of our study is the use of self-reported questionnaire to measure ELS events. This type of measure is prone to recall bias and may not provide a complete evaluation of ELS. Furthermore, our study did not disentangle the effect of each type of ELS experience, even though research shows that different adverse events may have different effects on brain structure and network connectivity. For example, deprivation and neglect are linked to changes in executive control network regions, such as the dorsolateral prefrontal cortex and parietal cortex, while threat and abuse-related exposures are linked to alterations in regions of the salience network [67]. Additionally, adults who grew up in poverty exhibit reduced activation in the ventrolateral prefrontal cortex and have difficulty regulating emotions [68]. In a recent study alterations in connectivity within the SN was found to mediate the effects of childhood abuse and neglect with problematic alcohol use [69]. Although there are no studies that have directly compared the impact of ELS on connectivity differences with increase in BMI measures in the AUD population, the age at which the stress occurred [70] and the level or duration of stress exposure [71] are crucial factors that should be explored in future studies. Moreover, the correlations observed with ELS and BMI measures with brain connectivity in individuals with AUD (vs. non-AUD) give rise to various conclusions. For instance, it is possible that ELS influences both alcohol abuse and excessive eating. Alternatively, it could be that ELS-driven AUD contributes to overeating, or that ELS-driven overeating increases vulnerability to alcohol overconsumption. The significance of these findings emphasizes the need for longitudinal studies instead of solely relying on cross-sectional research. It is also crucial to conduct longitudinal studies that follow individuals with AUD from an early stage, allowing the observation of potential changes in their brain patterns over time, particularly in relation to any fluctuations in BMI. Another potential limitation is that AUD treatment seekers were administered various medications during their in-patient stay, including anti-depressants, anti-psychotic agents, smoking cessation agents, antihypertensive agents or an attention deficit hyperactivity disorder therapy agent, which may have influenced the functional connectivity results (please refer to Table 1); however, since these were absent from the non-AUD control group, it was not appropriate to include these as covariates. Lastly, our identified connectivity patterns between SN seeds and other brain networks does not align with many other studies on the effects of ELS on SN seed-based connectivity. This may be attributed to the relatively low severity of the stressors reported in our cohort, making direct comparisons with previous results difficult. In our forthcoming study, we aim to investigate potential sex effects that may be influencing the observed correlations and relationships. Furthermore, it is crucial to replicate our findings using large datasets to assess the consistency and reliability of the results, reinforcing the significance and validity of our study’s outcomes.
Conclusion
To conclude, we identified positive correlations in the connectivity of our SN seeds with clusters associated with emotion, self-regulation, decision-making and impulsivity for salient stimuli in an AUD vs. non-AUD population which correlated positively with a history of ELS-related events and BMI measures. Further our results revealed the impact of the interaction of ELS events and BMI elevation on the observed brain connectivity patterns in study participants with AUD (vs. non-AUD). These findings underscore the significance of ELS and BMI in modulating the SN seed connectivity in AUD and its role in the neurobiological mechanisms that drive AUD. The results from our study suggest potential directions for future large-scale research on the neural mechanisms for the comorbid occurrence of obesity in individuals with AUD with a history of ELS. This might facilitate the development of targeted interventions for such individuals.
Data availability
The information analyzed in this study from the NIAAA is bound by specific licenses and restrictions. The dataset is under the care and control of the NIAAA Office of the Clinical Director and is securely housed there. Dataset access requests are directed to Melanie Schwandt, melanies@mail.nih.gov. The corresponding authors can provide the findings of this study upon receiving a reasonable request.
References
Gondré-Lewis MC, Warnock KT, Wang H, June HL Jr, Bell KA, Rabe H, et al. Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress. 2016;19:235–47.
Ramchandani VA, Stangl BL, Blaine SK, Plawecki MH, Schwandt ML, Kwako LE, et al. Stress vulnerability and alcohol use and consequences: From human laboratory studies to clinical outcomes. Alcohol. 2018;72:75–88.
Schwandt ML, Heilig M, Hommer DW, George DT, Ramchandani VA. Childhood trauma exposure and alcohol dependence severity in adulthood: mediation by emotional abuse severity and neuroticism. Alcohol Clin Exp Res. 2013;37:984–92.
Canessa N, Basso G, Carne I, Poggi P, Gianelli C. Increased decision latency in alcohol use disorder reflects altered resting-state synchrony in the anterior salience network. Sci Rep. 2021;11:19581.
Sullivan EV, Müller-Oehring E, Pitel AL, Chanraud S, Shankaranarayanan A, Alsop DC, et al. A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol Psychiatry. 2013;74:547–55.
Arcurio LR, Finn PR, James TW. Neural mechanisms of high-risk decisions-to-drink in alcohol-dependent women. Addict Biol. 2015;20:390–406.
Syan SK, McIntyre-Wood C, Minuzzi L, Hall G, McCabe RE, MacKillop J. Dysregulated resting state functional connectivity and obesity: A systematic review. Neurosci Biobehav Rev. 2021;131:270–92.
Sayon-Orea C, Martinez-Gonzalez MA, Bes-Rastrollo M. Alcohol consumption and body weight: a systematic review. Nutr Rev. 2011;69:419–31.
Boden G, Chen X, DeSantis RA, Kendrick Z. Ethanol inhibits insulin action on lipolysis and on insulin release in elderly men. Am J Physiol-Endocrinol Metab. 1993;265:E197–E202.
de la Monte S, Derdak Z, Wands JR. Alcohol, insulin resistance and the liver-brain axis. J Gastroenterol Hepatol. 2012;27:33–41.
Traversy G, Chaput J-P. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122–30.
Fazzino TL, Fleming K, Sher KJ, Sullivan DK, Befort C. Heavy drinking in young adulthood increases risk of transitioning to obesity. Am J Prev Med. 2017;53:169–75.
Colditz GA, Giovannucci E, Rimm EB, Stampfer MJ, Rosner B, Speizer FE, et al. Alcohol intake in relation to diet and obesity in women and men. Am J Clin Nutr. 1991;54:49–55.
He C, Fan D, Liu X, Wang Q, Zhang H, Zhang H, et al. Insula network connectivity mediates the association between childhood maltreatment and depressive symptoms in major depressive disorder patients. Transl Psychiatry. 2022;12:89.
Gupta A, Mayer EA, Acosta JR, Hamadani K, Torgerson C, van Horn JD, et al. Early adverse life events are associated with altered brain network architecture in a sex- dependent manner. Neurobiol Stress. 2017;7:16–26.
Elton A, Tripathi SP, Mletzko T, Young J, Cisler JM, James GA, et al. Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Hum Brain Mapp. 2014;35:1654–67.
Agarwal K, Demiral SB, Manza P, Volkow ND, Joseph PV. Relationship between BMI and alcohol consumption levels in decision making. Int J Obes. 2021;45:2455–63.
First MB Structured clinical interview for DSM-IV axis I disorders. Biometrics Research Department (1997).
First MB, Williams JB, Karg RS, Spitzer RL. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlingt, VA: Am Psychiatr Assoc. 2015;2015:1–94.
Compton WM, Dawson DA, Goldstein RB, Grant BF. Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug Alcohol Depend. 2013;132:387–90.
Sobell LC, Sobell MB Timeline followback: user’s guide. Addiction Research Foundation= Fondation de la recherche sur la toxicomanie …(1996).
Cohen RA, Hitsman BL, Paul RH, McCaffery J, Stroud L, Sweet L, et al. Early life stress and adult emotional experience: an international perspective. Int J Psychiatry Med. 2006;36:35–52.
Sokołowski A, Dragan W. New empirical evidence on the validity and the reliability of the early life stress questionnaire in a Polish sample. Front Psychol. 2017;8:365.
Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of Alcohol Withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84:1353–7.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101.
Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905.
Galandra C, Basso G, Manera M, Crespi C, Giorgi I, Vittadini G, et al. Salience network structural integrity predicts executive impairment in alcohol use disorders. Sci Rep. 2018;8:14481.
Grodin EN, Cortes CR, Spagnolo PA, Momenan R. Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug Alcohol Depend. 2017;179:100–8.
Padula CB, Tenekedjieva L-T, McCalley DM, Al-Dasouqi H, Hanlon CA, Williams LM, et al. Targeting the salience network: a mini-review on a novel neuromodulation approach for treating alcohol use disorder. Front Psychiatry. 2022;13:893833.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol). 1995;57:289–300.
Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addict Biol. 2017;22:206–17.
Han J, Keedy S, Murray CH, Foxley S, de Wit H. Acute effects of alcohol on resting-state functional connectivity in healthy young men. Addict Behav. 2021;115:106786.
Khalili-Mahani N, Zoethout RM, Beckmann CF, Baerends E, de Kam ML, Soeter RP, et al. Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo-controlled crossover study in healthy young men. Hum Brain Mapp. 2012;33:1003–18.
Maurage P, Masson N, Bollen Z, D’Hondt F. Eye tracking correlates of acute alcohol consumption: a systematic and critical review. Neurosci Biobehav Rev. 2020;108:400–22.
Poulsen MB, Jakobsen J, Aagaard NK, Andersen H. Motor performance during and following acute alcohol intoxication in healthy non-alcoholic subjects. Eur J Appl Physiol. 2007;101:513–23.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83.
Frewen PA, Dozois DJ, Neufeld RW, Densmore M, Stevens TK, Lanius RA. Neuroimaging social emotional processing in women: fMRI study of script-driven imagery. Soc Cogn Affect Neurosci. 2011;6:375–92.
Zhu X, Dutta N, Helton SG, Schwandt M, Yan J, Hodgkinson CA, et al. Resting-state functional connectivity and presynaptic monoamine signaling in Alcohol Dependence. Hum brain Mapp. 2015;36:4808–18.
Durkee CA, Sarlls JE, Hommer DW, Momenan R. White matter microstructure alterations: a study of alcoholics with and without post-traumatic stress disorder. PloS one. 2013;8:e80952.
Grodin EN, Lin H, Durkee CA, Hommer DW, Momenan R. Deficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by VBM: Effects of co-morbid substance abuse. NeuroImage Clin. 2013;2:469–76.
Momenan R, Steckler LE, Saad ZS, van Rafelghem S, Kerich MJ, Hommer DW. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. 2012;204:101–11.
Vuilleumier P, Armony JL, Clarke K, Husain M, Driver J, Dolan RJ. Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia. 2002;40:2156–66.
Guell X, Schmahmann JD, Gabrieli J, Ghosh SS. Functional gradients of the cerebellum. Elife. 2018;7:e36652.
Keren-Happuch E, Annabel Chen SH, Moon-Ho MH, Desmond JE. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp. 2014;35:593–615.
Jenness JL, Peverill M, Miller AB, Heleniak C, Robertson MM, Sambrook KA, et al. Alterations in neural circuits underlying emotion regulation following child maltreatment: a mechanism underlying trauma-related psychopathology. Psychological Med. 2021;51:1880–9.
Pilowsky DJ, Keyes KM, Hasin DS. Adverse childhood events and lifetime alcohol dependence. Am J Public Health. 2009;99:258–63.
Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacol (Berl). 2011;214:17–31.
Moustafa AA, Parkes D, Fitzgerald L, Underhill D, Garami J, Levy-Gigi E, et al. The relationship between childhood trauma, early-life stress, and alcohol and drug use, abuse, and addiction: An integrative review. Curr Psychol. 2021;40:579–84.
Robin RW, Chester B, Rasmussen JK, Jaranson JM, Goldman D. Prevalence, characteristics, and impact of childhood sexual abuse in a Southwestern American Indian tribe. Child Abus Negl. 1997;21:769–87.
Koss MP, Yuan NP, Dightman D, Prince RJ, Polacca M, Sanderson B, et al. Adverse childhood exposures and alcohol dependence among seven Native American tribes. Am J Prev Med. 2003;25:238–44.
Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. Eur Neuropsychopharmacol. 2013;23:24–32.
Cohodes EM, Kitt ER, Baskin-Sommers A, Gee DG. Influences of early-life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Dev Psychobiol. 2021;63:153–72.
Ilomäki M, Lindblom J, Salmela V, Flykt M, Vänskä M, Salmi J, et al. Early life stress is associated with the default mode and fronto-limbic network connectivity among young adults. Front Behav Neurosci. 2022;16:958580.
Corr R, Glier S, Bizzell J, Pelletier-Baldelli A, Campbell A, Killian-Farrell C, et al. Triple Network Functional Connectivity During Acute Stress in Adolescents and the Influence of Polyvictimization. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2022;7:867–75.
van Oort J, Tendolkar I, Hermans EJ, Mulders PC, Beckmann CF, Schene AH, et al. How the brain connects in response to acute stress: A review at the human brain systems level. Neurosci Biobehav Rev. 2017;83:281–97.
Colleluori G, Galli C, Severi I, Perugini J, Giordano A. Early life stress, brain development, and obesity risk: is oxytocin the missing link? Cells. 2022;11:623.
Entringer S, Buss C, Heim C. [Early-life stress and vulnerability for disease in later life]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2016;59:1255–61.
Kaufman D, Banerji MA, Shorman I, Smith ELP, Coplan JD, Rosenblum LA, et al. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes. 2007;56:1382–6.
Wang L, An C-X, Song M, Li N, Gao Y-Y, Zhao X-C, et al. Evaluation of childhood traumatic experience as a risk factor for alcohol use disorder in adulthood. BMC Psychiatry. 2020;20:15.
al’Absi M. The influence of stress and early life adversity on addiction: psychobiological mechanisms of risk and resilience. Int Rev Neurobiol. 2020;152:71–100.
Leng G, Adan RAH, Belot M, Brunstrom JM, de Graaf K, Dickson SL, et al. The determinants of food choice. Proc Nutr Soc. 2017;76:316–27.
Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–23.
Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci. 2008;1141:105–30.
Osadchiy V, Mayer EA, Bhatt R, Labus JS, Gao L, Kilpatrick LA, et al. History of early life adversity is associated with increased food addiction and sex-specific alterations in reward network connectivity in obesity. Obes Sci Pract. 2019;5:416–36.
Howell BR, Sanchez MM. Understanding behavioral effects of early life stress using the reactive scope and allostatic load models. Dev Psychopathol. 2011;23:1001–16.
McLaughlin KA, Weissman D, Bitrán D. Childhood adversity and neural development: a systematic review. Annu Rev Dev Psychol. 2019;1:277–312.
Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110:18442–7.
Rakesh D, Allen NB, Whittle S. Longitudinal changes in within-salience network functional connectivity mediate the relationship between childhood abuse and neglect, and mental health during adolescence. Psychol Med. 2023;53:1552–64.
Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
Danese A, Widom CS. Objective and subjective experiences of child maltreatment and their relationships with psychopathology. Nat Hum Behav. 2020;4:811–8.
Funding
Dr. Joseph is receiving support from National Institute on Alcohol Abuse and Alcoholism (Z01AA000135) and Nursing Research (1ZIANR000035-01), the Office of Workforce Diversity, the National Institutes of Health Distinguished Scholar Award, and the Rockefeller University Heilbrunn Nurse Scholar Award. KA has received an Intramural Research Training Award from the National Institute on Alcohol Abuse and Alcoholism, as well as a Fellowship from the Center on Compulsive Behaviors, National Institutes of Health, Department of Health, and Human Services. Please note that the authors are solely responsible for the content of this study, and it does not necessarily reflect the official views of the NIH. This study was in part funded by NIAAA Intramural program (ZIAAA000123, PI: Reza Momenan).
Author information
Authors and Affiliations
Contributions
Conceptualization—KA; Writing—Original Draft, KA; Data Analysis—KA; Data Interpretation—KA, RM, DG; Reviewing & Editing—KA, RZ, MS, VR, ND, DG, RM & PVJ; Supervision—DG & RM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agarwal, K., Joseph, P.V., Zhang, R. et al. Early life stress and body-mass-index modulate brain connectivity in alcohol use disorder. Transl Psychiatry 14, 43 (2024). https://doi.org/10.1038/s41398-024-02756-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-02756-8